Knowing the density of zinc metal is critical. It will help you decide whether the zinc metal is suitable for a specific use or not.
In this article, we explore all basic facts about zinc metal. From numerical figures, zinc alloy densities to factors affecting the density – you will find all information right here.
What is Zinc Density in kg/m3?
We can describe density as the mass per unit volume of zinc. In this case, zinc density is an essential criterion in identifying its application.
The density of pure metallic zinc is about 7140 kg/m³ under an ordinary room temperature of about 25°C. To break it down, it simply means that one cubic meter of it will weigh 7140 kilograms.
Just like every other metal, this density is not fixed and can vary depending on temperature and other factors. These include impurities present and any other additional alloying elements.

Comparing Molten Zinc Density to Zinc Dust Density
There exists a notable difference in the density of zinc when it is in its solid state, molten state, or when powdered. These variations are significant especially when you are looking at the form of the zinc you intend to employ in your application. Let us analyze how they compare:
· Molten Zinc Density
Zinc metal melts at temperatures above 419°C meaning its density becomes slightly lower than that of solid zinc. This density falls around 6570 kg/m³ at a temperature of approximately 450°C.
This decline in density is because metal atoms in the liquid state tend to be less crowded as compared to the solid state. Knowing such a reduced density is essential especially when you are dealing with galvanization using molten zinc to coat steel. The thickness of the coat heavily relies on this density.
· Zinc Dust Density
Powdered zinc has a comparatively lower density because of the air spaces between the powder particles. This means that they have a significantly lower bulk density compared to molten zinc.
Depending on the size of the powdered particles and how compact they are, this may range between 3500-4500 kg/m³. Knowledge of this density majorly assists manufacturers in determining the most economical transport and storage modes.
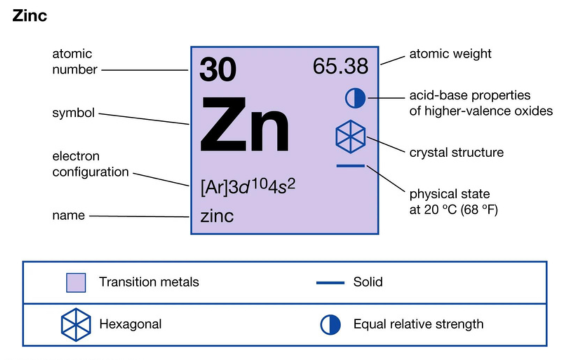
Exploring Zinc Alloy Density and Compounds
Zinc has an effective interaction with other metals and that is why we normally use it in alloys to enhance the metal characteristics for specific industrial uses. There is a variation between the density of such zinc alloys with that of the base zinc metal.
· Zinc Alloys
Some of the most common zinc alloys include:
- Zamak: this alloy series majorly contains zinc and aluminum with its density within the limits of 6600-6800 kg/m³. With excellent mechanical properties, we mostly apply it in die casting due to its moderately high density.
- Brass: this alloy has zinc and copper in its composition with its density ranging between 8300-8700 kg/m³ largely dependent on the proportion of the two metals. Since it is very strong and not easily corroded, we use it for electrical connectors, plumbing, and decorative item
Zinc Compounds
The capabilities of zinc combined with other elements are very high with such compounds exhibiting unique densities. Let us have a brief look at some of them:
- Zinc Oxide (ZnO): with a density of around 5606 kg/m³, we majorly use it in rubber production, and manufacturing cosmetics among many other applications.
- Zinc Sulfide (ZnS): though this compound has a low density of around 4090 kg/m³, it works very well with optical materials and pigments
It is essential, especially if you are an engineer or manufacturer, to have a very good grasp of the density of zinc alloys and their compounds. This is because they determine the weight, endurance, and function of the material.
How to Calculate Zinc Density
The general formula designated for calculating the density of any material, including zinc, is given by:
Density = Mass/Volume
To calculate the actual density of zinc, you first have to measure its mass in kilograms using a weighing scale. You then have to get the volume by multiplying its length, width, and height using the formula:
Volume = Length x Width x Height
However, this case may be different if you are dealing with molten zinc because you will have to factor in temperature change. This is because zinc is known to expand when heated and contract when cold.
Factors Affecting Density of Zinc
Just like any other physical characteristic of metals, the density of zinc is affected by several factors. Let us take a deep dive into some of them:
- Temperature: it has an inverse relation with temperature which means that any decrease in temperature increases the density of zinc. This is because as you melt your zinc, the distance between its molecular structures is relatively further apart hence lesser density.
- Alloy Composition: the density of the zinc material depends on the proportion of zinc and the other metals that form that alloy. For instance, combining aluminum to make zamak with zinc reduces the density of the material from that of pure zinc.
- Impurities: impurities present in zinc impact its density in such a way their presence makes the zinc less dense. Pure zinc usually has a very high density while that of lower purity will depend on the type and degree of impurities.
- Physical Form: just as we mentioned earlier, the density of zinc will depend on its state in either solid, molten, or powdered form. The bulk density of zinc powder is lower than that of solid due to the spaces between the particles.
Conclusion
If you wish to incorporate zinc into your business or industry, you must have an in-depth understanding of its density. It is through this that you can maximize the use of this metal by enhancing its efficiency, reducing your fabrication cost while at the same time enhancing the quality of your products.
Related information:
Melting Point of Zinc – Source: KDM
Does Zinc Rust – Source: KDM
Zinc – Source: BRITANNICA
Is Zinc Magnetic – Source: KDM




