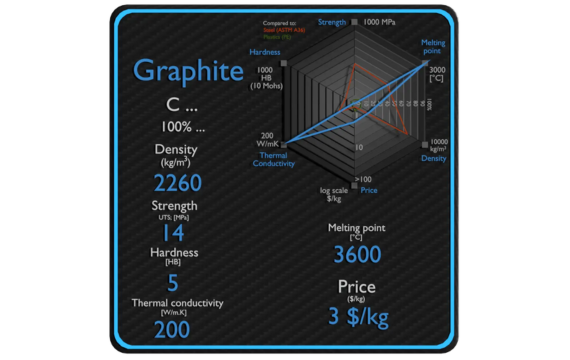
In this article, we will focus on density of graphite.
From the estimate values, how to determine the density to factors affecting graphite density – we shall explore all fundamentals aspects about this critical property.
Let’s dive right in:
What is the Theoretical Density of Graphite?
Theoretical density of graphite is close to 2.26 grams per cubic centimeter (g/cm3). Graphite has a regular arrangement of carbon atoms in hexagonal patterns, ultimately producing a stable planar bond but a weak interlinear force.
Such an arrangement is responsible for its lightweight character and rather low density when compared to other forms of carbon.
You will notice graphite’s density is important when choosing materials for use in the production of lubricants, electrodes, and products with heat resistance.
How to Measure Density of Graphite
Direct Measurement:
Mass: Take an accurate measurement of the mass of a sample graphite by means of a precision scale.
Volume:
- In the case of cubes and spheres, etc., the volume of these shapes is calculated by geometric formulas.
- For irregular shapes, use displacement methods:
- Archimedes’ Principle: This process involves immersing the graphite sample in a graduated cylinder containing water. The change in water level represents the quantity of sample in the measuring cylinder.
- Pycnometer: Take a pycnometer and put it into a liquid whose volume is known in advance. Introduce the graphite sample into the equipment and record the new volume added. The difference is the quantity of graphite embodied in the material.
Indirect Measurement:
- X-ray Diffraction (XRD): This technique can be used to find out the crystal structure and lattice parameters of graphite as well as estimate the theoretical density.
- Helium Pycnometry: This technique uses the amount of helium gas necessary to fill the pores of the sample to estimate its volume.
Considerations:
- Porosity: Graphite often has an irregular, porous structure, which might influence its documented density. The given approach may produce slightly different results from those obtained when using a different method.
- Sample Preparation: Make sure that the graphite sample is free from dust or moisture and is ready for measurement.
- Accuracy: Measurement accuracy is influenced by the type of tools used and how the operation is carried out.
Density of Various Types of Graphite
· Synthetic Graphite Density
When working with synthetic graphite, its density varies between 1.6 and 1.9 g/cm3. This graphite is developed based on the heat treatment of carbon structures, which leads to a material with uniform characteristics.
Synthetic graphite has a comparatively low density and is thus used in applications such as electrodes and batteries where weight is an important factor of consideration.
· Isostatic Graphite Density
Isostatic graphite normally exhibits a density of 1.72 to 1.9 g/cm3. This is a high-density material and is developed at the same pressure to the four cardinal points of direction for smoothness. You incorporate isostatic graphite into high-strength and accuracy applications such as semiconductors and high-temperature molds.
· Extruded Graphite Density
Extruded graphite has a density approximately 1.55 to 1.72 g/cm3. It is made by sintering carbon or graphite under pressure and passing it through a die, leading to elongated structures. Extruded graphite is used in furnace components, casting molds, and heat exchangers since it provides density for thermal conductivity and strength.
Factor Affecting Graphite Density
· Crystal Structure
When examining the density of graphite, you begin with the crystal structure of graphite. The graphitic structure of carbon atoms stacked in layers with covalent bonds within layers and weak van der Walls forces between layers influences density.
It is important that you observe that the manner in which these layers are arranged can differ with regard to the compactness of the material.
A perfect arrangement of layers of a crystal brings more density, while the arrangement of layers is relatively random, so less density is there. You can anticipate the way in which graphite’s organization affects its use in applications such as lubrication.
· Pore Size
What you realize is that the porosity of the graphite significantly defines aspects such as its density. Higher porosity means that there are more spaces that are open within the material, which means that the density of the material will be lesser.
When the pores are smaller or less in number, then the resin gets packed more densely and the overall density is higher.
The pore size can be controlled during the manufacturing process; thus, the graphite can be customized in a specific manner. For instance, higher-density graphite with little porosity is often used for electrodes, where strength and conductivity are required.
· Particle Size
When you compare different particle sizes, the density of the graphite changes, and this is something that you would notice. This is because parts with a small size pack more closely; hence, fewer and smaller voids exist in the pack. As for the bigger particles, they leave more voids in the structure, thus reducing the density.
Larger particles of graphite are used in applications that require higher density, such as heat resistance elements. Finer particles lead to better packing and improved performance of the materials.
Therefore, while using the graphite, by controlling the particle size, you manage to get a density of the graphite that is appropriate for your project.
· Molded Graphite
When comparing the physical properties of molded graphite, it is seen that the density level depends on the type of manufacturing process used. Molded graphite is made by applying pressure to graphite powder to form it into a specific shape. The pressure has an impact on the density of the particles.
Higher pressure leads to denser material, hence making it suitable in places where density matters most, such as strength and durability. Understanding how molding influences the density of graphite enables you to modify methods of production to fit specific needs to ensure that graphite.
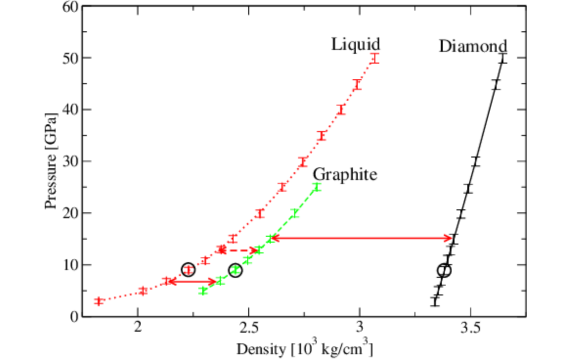
Comparing Density of Diamond and Graphite
Even though both are made up of carbon, the density of diamond is 3.5 g/cm3, while that of graphite is 2.26 g/cm3. The reason for this difference is in the atomic structures of the two forms.
Diamond is composed of three-dimensional covalent bonds that lead to the formation of a denser, stronger material. While graphite is composed of flat layers, and consequent characteristics of the material are lighter and softer but with unique electrical and thermal properties.
Diamond has a 3D tetrahedral structure, and every carbon atom in the structure is bonded covalently to four adjacent carbon atoms. This results in an extremely stable and tightly packed structure of formula units. The much closer packing of the atoms within the confines of this structure leads to a mass density of about 3.5 g/cm3.
Contrary to this, graphite has an ordered, two-dimensional layered structure. Carbon atoms are hexagonally arranged and strongly bonded by covalent bonds within the layers but weak intermolecular forces outside them.
The two layers are bonded by much weaker forces known as van der Waals forces, and this enables the layers to slide over each other.
This loose interlayer bonding gives it a much lower density than that of graphite, with the density of graphite ranging between 2 and 1.26 g/cm3. These weaker forces are the reason why graphite is soft and has lubricating properties, as the layers of carbon atoms can slide over one another very easily.
FAQs
What is High-Density Graphite?
High-density graphite grade is a type of graphite containing fewer pores and more closely packed carbon particles, thus imparting a high density above 1.85 g/cm3.
This density is attained through various manufacturing processes that aim at minimizing porosity and enhancing compaction of the particles of graphite.
It is stronger, has better electrical and thermal conductivity, thus making it suitable for applications of high reliability, such as in electrodes, molds, and heat exchangers.
How do you increase the density of the graphite?
· Vibration Compaction
You increase graphite density through vibration compaction because the application of mechanical vibrations facilitates the packing of carbon particles tightly. This results in lower porosity and consequently higher density of the material, together with enhanced mechanical characteristics.
· Impregnating Graphite with Resins
By impregnating graphite with resins, you fill its pores with liquid resins that set and thereby gain extra weight. This process increases the strength, wear resistance, and resistance to oxidation of graphite to make it ideal for several applications.
Conclusion
Graphite provides improved strength and wear resistance for harsh use conditions. You can make it denser through techniques such as vibration consolidation and resin infusion, enhancing its applications in different sectors.
More resources:
Density of Carbon – Source: KDM
Carbon Graphite – Source: KDM
Melting Point of Graphite – Source: KDM




