Metalloids are elements that possess characteristics of the metals and the nonmetals families.
In the periodic table, they are located in the area along the diagonal line dividing metals from nonmetals.
They possess properties of metals and non-metals at the same time.
Lists of Metalloids and their Applications, Physical and Chemical Properties
1. Boron (B)
Boron has the chemical symbol B and atomic number 5, and it is the lightest metalloid. You often encounter boron in two major forms: Amorphous boron, it is a brown powder and crystalline boron is in the form of black hard substance. It has a profound capacity to create several molecular structures and systems like clusters, chains, and networks.
Physical Properties of Boron
- State: Boron exists in two allotropic forms: There is the amorphous form which is brown powder and the other one is crystalline boron which is hard black color.
- Color: Amorphous boron has a brown color while crystalline boron has a black metallic-like lustrous appearance.
- Melting Point: Boron has a high degree of hardness, that is, it melts at a temperature of 2076°C, thereby putting it on the list of elements with the highest melting points.
- Boiling Point: The boiling point of boron is as equally high as 3927°C.
- Density: Density of boron depends with the form of boron in question. Amorphous boron has a density of about 2.34 g/cm³ and crystalline boron has a little higher density of 2.46 g/cm³.
- Hardness: You will discover that crystalline boron is relatively hard. It ranks around 9.3 on the Mohs hardness scale which makes it in the league of some of the hardest materials that are known.
- Conductivity: Boron is not a good conductor of electricity at room temperature but has semi-conductor properties at certain conditions.
- Crystal structure: Boron is known to crystallize in four modifications: α-rhombohedral, β-rhombohedral, α-tetragonal, and β-tetragonal.
![]()
Chemical Properties of Boron
- Reactivity: Boron is actually inert at ordinary temperatures and does not dissolve in most acids and alkalis.
- Oxidation: Boron burns at high temperatures with oxygen to give the formation of boron oxide (B2O3).
- Reactions with halogens: Boron is highly reactive and when heated reacts with halogens to form compounds known as boron halides including boron trifluoride (BF3).
- Reactions with metals: Boron happens to form borides with metals for example magnesium boride (MgB2).
- Acidity: Boron oxide and all its derivatives are quite acidic in characteristic.
- Covalent bonding: Boron commonly forms covalent bonds with other elements most of the time due to its tendency of being short of electrons.
Applications of Boron
- Glass and Ceramics: Boron is used in borosilicate glass which you understand is very resistant to thermal shock for instance Pyrex glass. You will also see it applied in high-temperature ceramics.
- Semiconductors: Boron has a specific role of doping within the semiconductor devices where the electrical properties of silicon are altered.
- Detergents and Bleaches: Boron compounds such as borax promote cleaning agents because they increase the effectiveness of water conditioner and stain-eradicating processes.
- Nuclear Reactors: In nuclear reactors, you see boron helps regulate nuclear reactions effectively by acting as an absorber of neutrons.
- Agriculture: Boron is an important micronutrient for plants. Different diseases of plants are caused due to boron deficiency and thus boron-containing fertilizers are utilized to enhance crop productivity and quality.
2. Silicon (Si)
Silicon is located on the periodic table with the symbol Si and it has an atomic number of 14. Silicon is a gray crystalline solid which is the second most abundant element in the Earth’s crust. It is present in the sand which is silica; rocks which are silicates; and most importantly it is in all the electronic gadgets you use.
Physical Properties of Silicon
- State: Silicon is a hard, brittle crystalline solid with a metallic glitter.
- Color: It is gray in color with a metal-like sheen, that makes it to have a shiny look.
- Melting Point: Silicon’s melting point is 1414°C.
- Boiling Point: The boiling point for it is much higher at 3265°C.
- Density: The density of silicon is 2. 33 g/cm³.
- Hardness: Silicon is moderately hard with the Mohs hardness ranging approximately around the value of 6. 5, though brittle which you will experience when touching Crystalline silicon.
- Electrical conductivity: Silicon is a semiconductor and this means that it has properties that are between that of metal and an insulating material.
Chemical Properties of Silicon:
- Reactivity: Silicon is quite inert at room temperature.
- Reaction with oxygen: Silicon is the element that combines with oxygen, to form silicon dioxide, a component of sand and quartz.
- Reaction with halogens: It reacts with halogens to form silicon halides for example silicon tetrachloride (SiCl4).
- Reaction with metals: Silicon reacts with metals to form silicides including magnesium silicide (Mg2Si).
- Acidity: Silicon dioxide is an acidic oxide which in reaction with base forms silicates.
![]()
Applications of Silicon
- Electronics: Silicon is the material of choice in the production of semiconductor devices. And you will come across it in nearly all the microchips, transistors, integrated circuits, and other electronics that are manufactured today.
- Construction: Silicon is extensively used in the manufacturing of concrete, ceramics, and glass. In construction material, silicon dioxide, which is a part of sand, facilitates in carrying out the duties.
- Medical Applications: Silicon-derived compounds such as Silicones are utilized in applications including medical implants, sealants, and lubricants.
- Solar Cells: Silicon is the most recommended material in photovoltaic cells, as they provide a conversion of sunlight into electricity.
3. Germanium (Ge)
Germanium is an element in the periodic table with the symbol Ge, and atomic number 32. It is a shiny metalloid that is hard and grayish-white in color. It is also typically applied in certain types of specialized electronics and optics, such as uses that involve detected infrared light.
Physical Properties of Germanium
- State: Germanium is a crystalline and is present mostly in solid form.
- Color: You will observe that it is shiny, and its color is grayish-white with a metallic sheen.
- Melting Point: Melting point of germanium is 938 °C.
- Boiling point: It has the highest boiling point that can go as high as 2833° C.
- Density: Germanium has a density of 5. 32 g/cm³.
- Hardness: Germanium is actually a brittle substance and is also not very hard, holding a Mohs hardness of 6.0 it is however quite fragile.
- Electrical conductivity: Germanium is a semiconductor that has intermediate electrical conductivity between the metal and non-metals, the insulators.
Chemical Properties of Germanium
- Reactivity: Germanium at room temperature is found to be much less reactive than most metals.
- Reaction with oxygen: Germanium reacts with oxygen by forming germanium dioxide, GeO2.
- Reaction with halogens: Like silicon, germanium also burns readily in excess of halogens and particular forms of germanium halide compounds like germanium tetrachloride (GeCl4).
- Reaction with metals: Germanium reacts with metals to produce germanides some of which include magnesium germanide (Mg2Ge).
- Amphoteric nature: Germanium dioxide is known to be amphoteric and thus can react with acids as well as bases.
Applications of Germanium
- Semiconductors: The use of germanium is prominent in high-speed electronics and more importantly in transistors and diodes. You will also come across it in specific semiconductor products intended for space use.
- Optics: Owing to its ability to transmit infrared light, germanium is used in infrared optics like lens of thermal imaging cameras.
- Alloys: This element is employed in the production of alloys to increase their strength and wear resistance, especially in phosphors for use in fluorescent lamps.
- Solar Cells: Germanium is also utilized in high-performance cells for solar cells, in satellites as well as other space facilities.
- Catalysis: Several germanium compounds are known to exhibit catalytic characteristics, and are used in many chemical reactions.
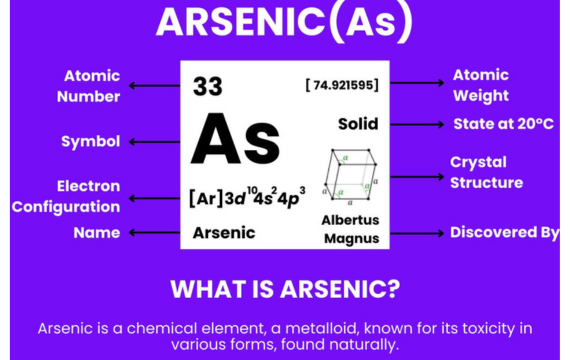
4. Arsenic (As)
The element Arsenic is denoted as As on the periodic table and has atomic number 33. Arsenic is a well-known metalloid which is a toxic element, but it has some uses in industry.
It exists in metallic form or as gray and non-metallic form which looks like yellow. Though highly toxic in nature, arsenic finds its application in a wide variety of electronics and alloys.
Physical Properties of Arsenic
- State: Arsenic is a semi-metal that crystallizes in a solid state and has several forms of allotropy, such as metallic gray and yellow.
- Color: Metallic gray is the most usual form of arsenic but it can also be in yellow when it is not a metal.
- Melting point: It also has a relatively low melting point of 817 degrees Celsius.
- Boiling Point: Arsenic has a sublimation point of 615°C, which means it has the ability to change directly from the solid phase to the gaseous phase. Which is without undergoing transformation to the liquid phase at normal atmospheric pressure.
- Density: Arsenic has a density of 5. 73 g/cm³.
- Hardness: The brittle and the relatively high hardness of arsenic are very high and get a Mohs hardness of approximately 3. 5.
- Electrical conductivity: Arsenic is an element that has extremely low electrical conductivity.
Chemical Properties of Arsenic
- Reactivity: Arsenic is not as reactive as you may think especially when at room temperature.
- Oxidation: arsenic oxide, for instance, the arsenic trioxide (As2O3) and arsenic pentoxide (As2O5), is formed through the reaction of the element with oxygen.
- Reaction with halogens: It forms halides by direct reaction with halogens as with halides of arsenic trichloride (AsCl3).
- Amphoteric nature: Arsenic oxides can show both acidic as well as basic character depending upon the reaction context.
- Toxicity: The arsenic compounds are very toxic in humans and animals regardless of the quantity present in the foods.
Applications of Arsenic
- Semiconductors: Gallium arsenide or GaAs is utilized in high-frequency devices, optoelectrical devices, and photovoltaic cells. You can especially apply it to LED and microwave technology.
- Wood Preservatives: For example, chromated copper arsenate (CCA) an arsenic compound is commonly used in the treatment of timber, but its use is limited today because of toxicity.
- Alloys: Arsenic is a powerful hardener of lead alloys and you can find them used in battery grids and cable sheathing.
5. Antimony (Sb)
Antimony is an element in the periodic table possessing an atomic number of 51 and is symbolized by Sb. It is a silvery-gray metalloid with a rather brittle consistency and uses antimony in flame retardants and alloys.

Physical Properties of Antimony
- State: The crystalline solid of antimony is lustrous, very brittle, and often in metallic color.
- Color: It has a signatory silvery gray shade with a shiny metallic look.
- Melting Point: Melting point of antimony is 631°C.
- Boiling Point: It has a boiling point of 1587°C.
- Density: The density of Antimony is 6. 68 g/cm³.
- Hardness: Antimony is a brittle and reasonably hard material possessing a Moh scale rating of about 3. 5-4. 5.
- Electrical conductivity:Antimony is not a good conductor of electricity.
Chemical Properties of Antimony:
- Reactivity: You will note that at Room Temperature antimony has a low reactivity.
- Oxidation: The reaction of antimony with oxygen produces some oxides of antimony such as antimony trioxide and oxide, Sb2O3, and antimony pentoxide, Sb2O5.
- Reaction with halogens: Antimony readily combines with halogens to form antimony halides for example antimony trichloride (SbCl3).
- Amphoteric nature: Antimony oxides can manifest both acidic and basic behaviour depending on reaction conditions.
Applications of Antimony:
- Alloys: Employ antimony to strengthen and give hardness and corrosion-resistant properties to a number of alloys. It is a usual part of lead-antimony alloys that are used in batteries, solder, and cable covering.
- Flame retardants: Antimony oxide is incorporated into plastics, textiles, electronics, etc., to enhance fire resistance and safety.
- Pigments: You can use antimony compounds as pigments in paints and plastics to impart heat-resistant colors.
- Semiconductors: Antimony finds its use as a basic element in the production of infrared detectors as well as special high-frequency electronics.
- Glass and Ceramics: Commonly used in Glass and Ceramic industries as an opacifier as well as a de-gassing agent.
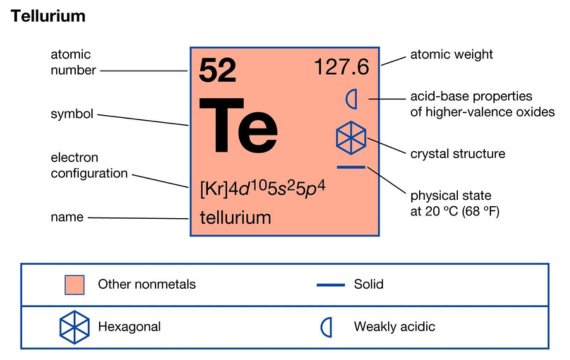
6. Tellurium (Te)
Tellurium is an element with the atomic number 52 and its symbol on the table is Te. Tellurium with the symbol Te is a rare and brittle metalloid with a silver-white colour and crystalline structure. It is associated with different minerals such as telluride ores.
Physical Properties of Tellurium:
- State: Tellurium is present in solid state and it is generally in the form of crystal.
- Color: With a silvery-white, lustrous solid-like metallic sheen, it is very shiny in appearance.
- Hardness: It has a relatively low hardness on a Mohs scale of around 2.5, which in essence tells you that it is fairly soft in nature.
- Melting point: The melting point is relatively low which is about 450°C.
- Boiling point: A fairly high boiling point of 990°C.
- Density: Density of Tellurium is 6. 24 g/cm³.
- Electrical conductivity: Tellurium has low conductivity of electricity but has some aspects of a semiconductor.
Chemical Properties of Tellurium:
- Reactivity: Tellurium is rather nonreactive at room temperature
- Oxidation: Tellurium undergoes oxidation with oxygen to produce tellurium oxides for instance tellurium dioxide (TeO2).
- Reaction with halogens: Like any other element, tellurium interacts with halogens to create compounds known as tellurium halides including tellurium tetrachloride (TeCl4).
- Amphoteric nature: Tellurium oxides are known to display both acidic and basic behaviour depending on the conditions that are prevailing at the time of the reaction.
Applications of Tellurium:
- Semiconductors: Tellurium is used as semiconductors for electronics, particularly in photovoltaic cells, thin-film solar cells, and infrared detectors.
- Thermoelectric Devices: Tellurium finds application in thermoelectric technology that enables direct conversion of heat into electricity and is used in refrigeration and power-producing modules.
- Rubber and Glass: Compounds of tellurium are employed in the production of rubber as vulcanizing agents as well as colorants for deep red and blue glass.
- Metallurgy: Tellurium is employed in certain metallurgical procedures since it enhances the qualities of the metals.
- Alloys: Tellurium enhances the ease of machining and increases the strength of lead and steel that are used in making batteries and cable sheaths respectively.
7. Polonium (Po)
Polonium is when denoted using the symbol Po and it has an atomic number of 84. Polonium is an extremely radioactive metalloid that is rarely found and was identified by Polish-born French physicist Marie Curie in 1898. It is highly radioactive and for this reason, it is highly hazardous and should be handled with a lot of precautions.
Physical Properties of Polonium
- State: Polonium is a solid at standard conditions.
- Color: It has a silvery–gray metallic luster.
- Density: Polonium of course has a density of 9. 2 g/cm³
- Melting Point: The element polonium has a melting point of 254 °C.
- Boiling Point: Polonium has a boiling point of 962°C.
- Electrical Conductivity: Polonium is able to conduct electricity like a metal and like other metals within the solid phase of matter.
- Radioactivity: Polonium is an extremely radioactive element that discharges alpha rays. Its most stable isotope is polonium-210 which has a half-life of 138 days.
Chemical Properties of Polonium
- Reactivity: Polonium can readily react with oxygen in the air forming a dioxide polonium oxide (PoO₂). Besides, it is capable of undergoing direct reaction with halogens to form polonium halides such as polonium chloride (PoCl₂).
- Oxidation States: Polonium has a tendency of showing an oxidation state of +2 and +4 in the way it combines with other elements.
- Toxicity: Polonium is a hazardous substance because of its radioactivity and even a small amount of it can be lethal. You will require special equipment to appropriately take care of it.
Applications of Polonium
- Antistatic Devices: Polonium-210 is used in antistatic devices where it eliminates static electricity for applications such as photography, paper processing, and textile industries among others.
- Nuclear Batteries: You will find polonium in nuclear batteries. Whose radioactive decay which releases heat is then converted into electrical energy for use in space travel and weaponry.
- Neutron Sources: Polonium combined with beryllium is a neutron source that you will meet in experiments and some early nuclear weapons.
- Spacecraft Heating: In spacecraft, polonium is used to produce heat to ensure that instruments work effectively in cold temperatures especially where the spacecrafts are deep in space.
Conclusion
As you can see, we only have 7 metalloids in the periodic table. Each element has unique property that makes them suitable for very specific applications.
Depending on your specific application requirements, KDM team will help you choose the right material for any application.
Related content:
Melting Point of Metals – Source: KDMFAB
Periodic Table – Source: RSC




