Having a better understanding of the melting points of different metal oxides is crucial. This is especially so if you are in the metal manufacturing or fabrication industry.
The melting point of a metal refers to the temperature or heat level at which a metal’s structure transitions from a stable solids form to a molten or liquid form
This guide provides valuable insights into why melting points are critical, the melting points of common metals, scientific measurement techniques, factors affecting metal melting points, and industrial applications.
1. Why Melting Points of Metals Is Significant?
The melting temperature of a metal is a factor when considering its industrial application properties.
This is because it determines the temperature level or stage at which you can use a particular metal without worrying about its failing risks.
Here are the sectors where you will find the melting points of different metals of great significance.
1.1 Melting Points of Metal In Manufacturing Processes

When you are in the manufacturing sector, your knowledge of the melting points of metals or alloys will guide you in setting different processing temperatures for different metals or alloys during their production.
1.2 Melting Points In Identifying and Classifying Metals
Data on the melting point of metals helps in their identification and classification. Melting points enables you to distinguish different metals.
You can also compare different alloys and identify their compositions with ease.
1.3 Selection of Materials and Products Design
Your knowledge of metals melting points will guide you in selecting the most suitable material for your applications.
For instance, if you desire a metal component for a high-temperature environment like a furnace, you must choose metal materials with desirably high melting points
A metal’s melting point will also guide you when designing your metal parts. For instance, the larger and thicker your design is, the longer it will take to melt completely.
1.4 Melting Points of Metals For Quality Control
As a metal fabricator or manufacturer, you can rely on the melting points of metals as a quality control measure.
If you apply too much heat to a metal compound material during processing, you can take note of the excess temperature and compare it with its standard melting point.
You can then use this information to assess if the overheating has caused damage to the metal.
2. The Melting Temperatures of Common Metals & Alloys
Melting Point Of Different Metals
The melting point of a metal or alloy determines its production process and its final application area.
Below is a list of some of the most common metals compounds and alloys you will come across in the manufacturing industry
2.1 Table Showing Melting Points of Common Metals And Alloys
| No | Metal | Melting Temperature | |
| Degrees Celcius (℃ ) | Degrees Fahrenheit (℉ ) | ||
| 1 | Aluminum | 660.3 | 1220.54 |
| 2 | Beryllium | 1287 | 2348.6 |
| 3 | Cobalt | 1495 | 2723 |
| 4 | Copper | 1084.62 | 1984.316 |
| 5 | Gold | 1064.18 | 1947.524 |
| 6 | Iron | 1538 | 2800.4 |
| 7 | Lead | 327.5 | 621.5 |
| 8 | Magnesium | 650 | 1202 |
| 9 | Nickel | 1453 | 2647.4 |
| 10 | Osmium | 3045 | 5513 |
| 11 | Platinum | 1772 | 3221.6 |
| 12 | Silver | 961.78 | 1763.204 |
| 13 | Tin | 232 | 449.6 |
| 14 | Titanium | 1668 | 3034.4 |
| 15 | Tungsten | 3422 | 6191.6 |
| 16 | Uranium | 1132 | 2069.6 |
| 17 | Vanadium | 1910 | 3470 |
| 18 | Zinc | 419.53 | 787.154 |
| Alloys | |||
| 1 | Brass | ||
| 2 | Bronze | 900-1100 | 1652-2012, |
| 3 | Mild Steel | 1370-1510 | 2498-2750 |
| 4 | Stainless Steel | 1400-1450 | 2552-2642 |
| 5 | Steel | 1370-1511 | 2498-2751.8 |
2.2 Chart Showing Melting Point of Metals
3. Factors Affecting The Melting Point of Metals
The melting point of metal represents the temperature at which metal will begin to melt.
You may wonder if this temperature should remain constant under all conditions.
Certainly not.
The melting points of pure metals are influenced by the following:
3.1 Atomic Structure of Metal
Metal elements are made of atoms with a specific number of protons and electrons. The electrons occupy the outer shells of atoms. They help atoms to bond with each other to form metallic lattice structures.
And so, the more electrons a metal atom has, the stronger the atoms will bond with each other, and the higher the metal’s melting point.
3.2 Atomic Mass of a Metal

The atomic mass of a metal refers to the number of positively charged particles called protons and the number of neutral particles called neutrons that the metal has.
Protons and neutrons of a particular metal have similar masses.
How then do they affect your metals’ melting points?
If you have a metal with a heavier atomic mass, it means the metal has more protons and neutrons and consequently, more electrons on its outer shell.
And because electrons facilitate the bonding between atoms, it means the higher the atomic mass, the more heat will be required to break the bond. So, your metal will have a higher melting point.
3.3 Metalic Bonding
Metallic Bonding and its properties
Did you know that for metals, electrons in the outer shell of their atoms, also called valence electrons, can be delocalized?
This allows electrons to move freely throughout the metal’s lattice structure to create stronger metallic bonds between the metal’s atoms.
The more electrons can be delocalized from a metal atom, the stronger the metallic bonds, and the higher the metal’s melting point.
3.4 Crystal Structure & Grain Size of Metals
In a solid state, metal particles are arranged in either crystalline or amorphous structures. The crystalline structure of a metal determines its melting point.
Metals with larger grain sizes allow for more bonding between atoms and, therefore, have higher melting points compared to those with smaller grain sizes.
3.5 Influence of Temperature
As you increase a metal’s temperature through heating, its atoms receive more energy. The atoms then begin to vibrate until the forces that bind them together are weakened.
With more heating, the atoms gain optimal energy to break free from each other and the metal begins to melt.
3.6 Influence of Pressure
For certain substances like ice, an increase in pressure lowers their melting point. However, for metals, an increase in pressure increases the melting point of the metal.
3.7 Level of Impurities
If you have pure metals then their melting points will be definite.
However, when you expose your metal to impurities or contaminants, the bonds forming the metal’s normal lettuce structure will be weakened during heating.
As a result, the melting point of the metal will be reduced as the bonds binding the metal atoms will be easily weakened and broken.
3.8 Alloying Compositions
A pure metal that has a regular atom structure with uniform strong bonds between them.
However, in alloying composition, different elements of different atom sizes are introduced thereby weakening the bonds between the atoms.
As a result, alloys have lower melting points than that of the individual metals that constitute them.
4. How to Determine Melting Points of Metals in the Lab
If you have a sample or samples of metals, you may wish to accurately measure their melting points either for quality control during processing or for research purposes.
Here are the main laboratory techniques you can adopt
4.1 Thermogravimetric Analysis (TGA)

The thermogravimetric measuring technique involves subjecting your metal sample to controlled heating inside a programmable furnace and in a controlled environment with a sensitive weighing scale.
The scale monitors the changes in the metal’s mass against time or temperature changes. There can either be an increase or decrease in the mass of the metal as temperature increases.
You can then analyze the generated data to determine the metal’s melting point.
4.2 Differential Scanning Calorimetry (DSC)

The differential Scanning Calorimetry technique helps you to measure changes in heat to determine your metal’s melting point. How does it work?
You heat your metal sample in a controlled atmosphere and record changes in temperature. You then analyze the recorded changes to determine the melting point of your sample.
The main advantage of this technique is that you can use it with thermally stable metal that can be heated at elevated temperatures.
4.3 Hot Stage Microscopy(HSM)
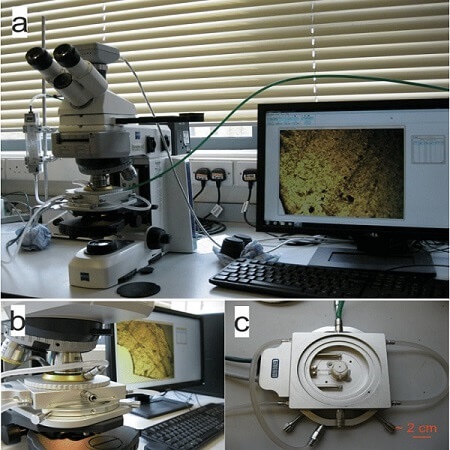
This technique of measuring a metal’s melting point involves observing and recording the changes under a powerful microscope as the metal sample gets heated.
The changes noted in the metal’s structure and shape. These changes help in determining the melting point of the metal.
You can use this technique to measure small temperature changes that may not be possible with other methods such as TGA or DSC.
5. Applications of Metal Melting Points in Different Industries
Below are some of the key sectors where the knowledge of metals melting points is applied.
5.1 Welding and fabrication industries

Your knowledge of metal melting points is important if you are in the welding or fabrication sector.
The material you choose, the welding technique you adopt, the equipment you select, and the temperature levels you set, are all guided by the melting point of the metal you plan to work on.
Making the right choice ensures your welds remain strong, durable, and of high quality.
5.2 Metallurgy and Manufacturing Processes

If you are in the metal manufacturing sector, you will appreciate the role your knowledge of the melting points of metals will play in your manufacturing processes.
During metal extrusion, casting, or forging processes, a better understanding of the metal’s melting points is critical.
To produce quality parts or components through such processes, you must choose the right material and determine the right melting temperatures.
5.3 Aerospace and Defence Industry

During flights, some metal components of an aircraft are subjected to massive pressure and temperatures.
For such parts to tolerate extreme heat conditions and pressure, their melting temperatures and strength must be much superior.
That is why you will find Titanium and its alloys used in the manufacture of aircraft due to their super high melting points and other excellent properties.
5.4 Automotive Industry

In the automotive industry, different metal parts are used. These parts have different melting points based on the set standards.
A car exhaust system, for instance, is made of Stainless steel whose melting point is high. An engine’s cylinder head is made of aluminum metal and has a low-melting point.
So during parts production, your understanding of the metal melting points of different metals will help you avoid defects resulting from overheating.
5.5 Metals Melting Points in Jewelry Making

To industrially craft catchy jewelry products and accessories, mastering the melting points of your metal materials is crucial.
Common jewelry-making metals like silver, gold, and Stainless steel, have different melting points.
And so, as a jeweler, to produce captivating and flawless products through casting or soldering processes, you must determine and control melting points accordingly.
5.6 Metal melting points in chemical research and development processes
As a researcher studying different metals, your understanding of metals’ melting points can guide you in creating new alloys for various industry applications.
Further, equipped with a better understanding of the boiling point of different metals, you can easily venture into other fields of research in the electronics sector, renewable energy sector, or medical sector.
6. Conclusion
Metal melting point refers to the energy required to transform a solid metal into a liquid.
This information is significant in identifying and classifying metals.
As a manufacturer or metal fabricator, your understanding of the melting points of metals, and factors affecting them is vital.
The knowledge helps you determine the most suitable temperatures for various manufacturing or fabrication processes. It also ensures that your products effectively fulfill their industrial applications.
There are various laboratory techniques that you can adopt to measure the melting points of different metals for quality control analysis or your research undertakings—request the Melting Points of Metal PDF for more information.




