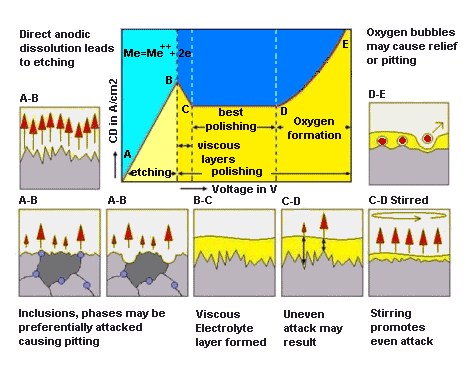
Electrolytic polishing is a metal finishing process used on metals like stainless steel and aluminium.
This guide covers the basics of electro polishing process, discussing its benefits and importance with a goal to help you understand electrolytic polishing and provide helpful tips for doing it correctly.
What Is Electrolytic Polishing?
Figure 1 – Electrolytic Polishing
Commonly used in metalworking and electrolytic chemical polishing of metals processing, the process of electrolytic polishing and etching machines utilizes electrical current to etch onto material electrodes.
An oxidizing agent can be applied during the procedure that provides conformal coating or corrosion protection. A DC voltage connects the anode and cathode. Electric current flows between them causing metal ions to be released from the working surface.
Molecular-level changes occur creating a mirror finish with the aim of creating a smoother and more appealing surface.
Also, it improves corrosion resistance and dimensional accuracy and is done by removing microscopic burrs or layers. Many materials can be treated this way, such as stainless steel, silver, zinc, and titanium.
Technical properties improved include dimensional accuracy, surface roughness, and cladding materials. Electrolytic polishing has advantages and disadvantages.
How Electrolytic Polishing Works?

Figure 2 – Electrolytic Polishing Working System
Understanding the process helps with finishing and manufacturing products. We will discuss the needed steps of electrolytic polishing below.
Pre-Treatment:
Metal surfaces are prepared by removing dirt and grease with a degreasing solvent and cloths. For best results, substances must be removed entirely and electrodes then connected to the metal surface.
Anodic Reaction:
Anodic dissolution is used. Electrical current is injected into the metal surface via electrodes that forms a chemical reaction with certain substances in the material.
An electric voltage is created between two different points. Electrons move from the negative anode to the positive cathode, and flow through the metal surface and react in a process called electrolysis.
Oxidation:
Oxidation occurs when oxygen attaches to atomic particles on the metal surface and increases electrical conductivity that allows for more particles to be removed during the process.
Air-reactive metals usually do not have this issue. Keep this in mind when dealing with specialized items.
Removal:
Electrochemical reaction occurs between attached elements and electrodes. Ions are released during electrolysis. Positive or negative particles are removed, and current helps them attach to the opposite electrode.
Moreover, they find a way out from the surface we’re working on to free unwanted substances and aim for the best results in electro-polishing and metal finishing. Electrons moving through the metal surface create a brilliant finish.
Rinsing:
Metal surfaces must be thoroughly rinsed. Use de-mineralized water to clear away remaining ions as it completes the electro-polishing cleaning stage. Allow a few minutes for drying before continuing.
Protective Coating:
In some cases, a protective coating is necessary that protects the metal surface from oxidizing and corrosion. Under high temperatures or humidity, exposing substances to electro-polishing increases their electrical reactivity.
For industrial and medical purposes, the coating is best practice – for example, in the dental field it assures treated areas retain quality properties.
Inspection is Important:
Electrolytic polishing process concludes with a visual inspection. Remaining particles or impurities are assessed in the metal surface.
Pre–treatment is important for most materials and metals, as the inspection detects if initial specifications have been met. Desired properties post electro-polishing are checked. Examples are improved surface finishes, smoother edges, or enhanced brightness.
Once outcomes meet the criteria, the process are considered finished for metal surfaces needing finishing.
| Step | Description | Example Materials/Uses | Importance |
| 1. Pre-treatment | Metal surfaces are prepared by degreasing and electrodes attached. | All types of metals | Ensures proper cleaning and electrode connection for effective electro-polishing. |
| 2. Anodic Reaction | Electrolysis is anodically dissolving material through electrical current injection from electrodes. | All types of metals | Facilitates the removal of unwanted substances, resulting in a smooth and polished surface. |
| 3. Oxidation | Oxygen increases electrical conductivity, enabling particle removal. | Air-reactive metals | Enhances precision, performance, and appearance of the electro-polished surface. |
| 4. Removal & Rinsing | Ions released during electrolysis are removed and surfaces rinsed with demineralized water. | All types of metals | Ensures a clean and brilliant finish that resembles a mini-electro-plating process. |
| 5. Protective Coating | Protect metal surface from oxidation, corrosion with a coating – especially under adverse conditions. | Industrial and medical applications | Preserves the quality of the treated metal surfaces, preventing corrosion and maintaining desired properties. |
| 6. Final Inspection | Inspect particles/impurities to ensure specs & desired properties met, concluding process. | All types of metals | Verifies that the electro-polishing process has successfully achieved the intended results. |
Electrolytic Polishing Process Steps
Key Factors That Affect The Polishing Process!
Many of us know that electrolytic polishing is a unique process. Compared to other metal finishing processes, it’s different. Its name suggests that it uses direct electrical current and the current passes between an anode and cathode.
However, the metal surface of a workpiece breaks down and precision finish is achieved, which cannot be accomplished through abrasive techniques alone. What factors should be considered for electrolytic polishing? Let’s explore some of them.
§ Chemical Composition Of Electrolyte Solution
Chemical composition is important for the electrolyte solution, as it is a core parameter which affects the uniformity and quality of the electro polishing surface finish.
Too little amount can result in longer polishing times as well as too much can cause metal surface corrosion.
Excessive chemical concentration can cause potential issues, and the ideal mixture for this depends on the workpiece material. For stainless steel, hydrochloric acid with 1-2 gram additives suffices.
§ Temperature Of Electrolyte Solution
Temperature plays a significant role in the polishing process, as it matters for the electrolyte solution. Above room temperature is effective for metal finishing. However, too high temperatures can cause electrical breakdowns.
Operational hazards may arise from electrolytic decomposition. Commercial electro-polishing machines have temperature control. Adjustments can be made during polishing and up to 30 Celsius degrees are ideal for achieving satisfactory outcomes.
Desired temperature levels vary with materials. Frequent checks are recommended to avoid hazards and a certified supplier is best for a top–level polishing process.
§ Current Density Applied
Current density applied in the range of 2 to 5 amperes per square decimetre is crucial as it affects both quality and time of the polishing process. Manufacturers must design lower power supplies.
AC type is preferred, and effectively divided cells for AC/DC power supplies are useful. We recommend precise machines from a reliable supplier because the current density depends on the size of anode cathodes.
Measuring and assessing sizes before starting saves time and ensures accurate finished products.
§ Surface Area Of The Workpiece
Surface size is crucial in electrolytic polishing. Bigger surfaces need higher current energy levels. Smaller surfaces require lower outputs. Batch size depends on factors like current density distribution. Upgrading area adds thickness.
Not to mention, the number indicates the size of metal components in the process, with greater surface areas needing more time to complete jobs but lesser areas requiring less time. Calculate the total area covered to determine time taken.
Manufacturers often find the right balance between efficacy and product quality, taking into consideration the product type and intended usage.
§ Material Of The Workpiece
Material is important for the overall polishing process. Different metals take different times to polish. Stainless steel is a popular choice due to its low carbon content, making it suitable for electro–polishing.
Brass alloys and iron–nickel combinations, although less suitable, have their own operational pros and cons.
Stainless steel might need less manual area coverage, but brass might need more coverage. Material choice is essential for efficiency and finish.
§ Duration Of The Electrolytic Polishing Process
Duration depends on specific processing parameters. For electro-polish stainless steel, time depends on current density applied. Uneven energy distribution leads to longer process times and may require more manual involvement.
Proper electrode placement and design ensure maximum output. Distribute current equally on the entire surface area. Direct circulation can be more effective that dispels potential buildups or layered processes.
Properly selected machines lessen the process duration. You should take into consideration the main factors, accuracy of stirring, agitation and mechanical design when making decisions.
§ Stirring/Agitation Of The Electrolyte Solution
Electrolyte Solution ensures accuracy during the electro–polishing procedure, and stirring the chemical bath often is important as it allows even electrical current distribution.
Without obstruction, current reaches all surface parts and stirring removes accumulating hydrogen bubbles that result from electrolysis which can disrupt the continuous system process.
| Factor | Importance | Ideal Conditions or Recommendations |
| Chemical Composition of Electrolyte Solution | Determines uniformity and quality of the surface finish | Use 1–2g additives in hydrochloric acid with stainless steel; Avoid over-concentration to prevent corrosion. |
| Temperature of Electrolyte Solution | Influences polishing process efficiency and safety | Maintain 30°C; Adjust temp. per material; Frequent checks for safety; Use certified supplier for best polishing |
| Current Density Applied | Affects quality and time of the polishing process | 2–5A/dm², AC supplies; Precise machines; Reliable suppliers; Measure & assess anodes/cathodes before start. |
| Surface Area of the Workpiece | Determines energy levels needed for polishing | Bigger surfaces: higher current energy levels; Smaller surfaces: lower outputs; Calculate total area to determine polishing time. |
| Material of the Workpiece | Affects overall polishing process, efficiency, and finish | Material choice important for efficiency and finish; Stainless steel: low carbon content suitable for electro–polishing. |
| Duration of Electrolytic Polishing Process | Dependent on processing parameters | Time and output depend on current density, electrode placement & design, equal distribution of current across surfaces area; use suitable machines to reduce process time. |
| Stirring/Agitation of the Electrolyte Solution | Ensures accuracy during the electro-polishing procedure | Stir chemical bath often for even current distribution and removal of hydrogen bubbles; Use high-intensity stirrer for best results; Results vary by workpiece material/design. |
Key Factors Affecting the Electrolytic Polishing Process
Materials And Equipment Needed For Electrolytic Polishing Method!
Electrolytic polishing needs specific materials and equipment to ensure quality results. Automatic electrolytic polishing and etching machine, understanding the requirements can save time. Here is a list of essential materials and equipment:
Electrolytic Polishing Machine:
Electrolytic Polishing Machine is a vital piece of equipment for this process because it provides voltage for creating a current which accelerates electrochemical reactions with metal parts.
Use one with proper capacity and regulation. In 2020, the electro polishing machine price was around €1400. Higher–priced models offer better results and save time compared to manual polishing.
Power Supply:
Power supply controls and adjusts current flow. Different types of output control options are available which vary in cost effectiveness. Use DC-based power sources with adjustable voltage.
Electrolytic Polishing Solution:
It‘s a chemical solution that serves as an electrolyte, and its composition depends on the parts being polished; containing acid and oxidizing agents such as chromates or nitrates.
Concentration levels depend on several factors. Higher concentrations yield better results. Choose a solution with a balanced pH.
Stainless Steel Polishing Wire Or Electrode:
An electrode acting as the cathode during an electrochemical reaction transfers electrons for the process, also known as a cathode.
Also, it controls potential, movement, and flow rate of electrolyte while stainless steel wires range from 0.25mm to 3.0mm in diameter.
Stainless wire electrodes provide good electrical current distribution and offer corrosion resistance in the electrolyte.
Glass Or Plastic Container For Holding Solution:
A container big enough to fit the parts for polishing should be resistant to the electrolyte and corrosion by–products.
Container should be deep and narrow, which helps maintain interaction between parts in the solution. For prolonged use, it needs to be non–conductive and an airtight seal is necessary.
Water Source For Rinsing:
Electro-polished parts need proper rinsing. Use a fresh water source for rinsing. Rinsing removes debris and electrolyte residues.
Using deionized or distilled water to prevent minerals and chemicals from contacting the parts results in enhanced performance.
Gloves And Protective Eyewear:
Safety is vital during electro-polishing. Wear gloves for protection against harmful contaminants. Protective eyewear shields your eyes, and prevents hazardous splashes. Eyewear is needed when handling materials and equipment.
Abrasive Paper Or Cloth:
Abrasive paper or cloth can smooth stainless steel surfaces and prepare the surface for optimal results.
Buffing Wheel:
Consider a buffing wheel for final touches. Though it is optional but can be useful for refining already-polished parts. Use it with polishing compound to even out residues, and a more aesthetic look. Some models have two buffing wheel sizes for efficiency.
Metal Or Plastic Tongs:
Metal or plastic tongs are useful during electro-polishing as they ensure easy and safe handling as well as reduce contact between the part and your skin.
Timer Or Stopwatch:
A timer is necessary for electro-polishing for measuring time intervals accurately. Time intervals depend on metal type, size, and shape. Concentration levels affect timing too. An accurate timer saves time in the long run.
Cleaning Solution For Final Clean:
Clean electro-polished parts with a mild solution. Use alcohol or diluted vinegar. Proper cleaning is crucial for optimal results, because it removes remaining residues. Clean parts before using them in further applications.
Steps To Electrolytic Polishing!

Figure 3 – Steps of Electrolytic Polishing
Learn about materials and tools. Understand the process. Electrolytic polishing removes marks forming a uniform surface that works on small-to-medium items, for example, jewelry and automotive parts. Follow these steps for perfect polish:
§ Learn Electrolytic Polishing Basics
Use a voltage supply and electric current. Select acid or alkaline solutions that will enable electrochemical changes on a workpiece.
Electrolytic polishing is used to improve the appearance of raw components. Use electro-deposition. Raise burrs or smooth discoloration from heat treating.
§ Prepare The Workpiece
Degrease workpieces with nonionic detergent. Use an ultrasonic cleaner. For best results, rinse with water and then dry to prevent air bubbles.
Substrate material and processing vary case–by–case, so it is important to recognize this. Consult specific instructions before dipping or electroplating.
§ Choose The Electrolyte Solution
Select the electrolyte solution carefully. Not all solutions suit every workpiece; it is important to recognize the difference. Sometimes, an alkaline solution is better. Other times, an acid solution works best. To strip chrome, use an acid-based electrolyte.
§ Pick The Anode Material
Consider the cathodic element. There are many anode materials, such as stainless steel, titanium, and other alloys.
Anodic material affects electrical charge, and chrome–plated anodes carry more current than titanium to passivate the surface.
§ Set Current Density And Temperature
Current density, which determines the electric current in an electrolyte, is electrical resistance per square inch and is measured in mA/sq inch.
Keep it constant for a uniform finish and choose the right temperature as warmth increases efficiency. Don’t exceed limits to avoid ruining the surface.
§ Connect The Workpiece And Anode To The Power Source
First, connect the power source ends to the anode and workpiece. One end will be positive, the other negative.
Before polishing, remember that more current increases electrolysis. Find a comfortable current range to avoid surface discoloration and imperfections.
§ Monitor The Polishing Process
Check the workpiece under microscopy regularly. Assess if corrosion is uniform or if some areas need more attention.
Use a microscope designed for quality control purposes. Regular microscopes may lack the power to see electroplated parts.
§ Rinse And Dry The Polished Workpiece
Remove polishing solution traces from workpieces and dry them in a dust-free, secure environment.
A regular wash-in between stages can limit cross contamination risk that helps electro polish stainless steel components without visible imperfections.
§ Inspect And Evaluate The Polished Surface
When satisfied, take a moment to assess the polished surface’s brightness. Decide if the finished product meets requirements.
Periodically monitor and familiarize yourself with desirable specifications in order to pursue perfection on the professional–grade, electrolytic polished finish!
Troubleshooting Common Issues!
Working on electrolytic polishing projects and troubleshooting common issues as they arise allows for progress to be made.
Identify, detect, and correct errors to save time and money; follow these tips for EP troubleshooting.
Check Electrolyte Composition And Condition.
Ensure the electrolyte is fresh and particle–free to ensure accurate results. You should measure pH levels and specific gravity. Replace after 30-50 cycles or if exposed too long.
Inspect Equipment And Materials.
Ensure proper functioning before starting a project. Check the tank, power supply, and electrodes. Look for surface wear.
Verify Power Supply Settings.
The setting impacts material size, surface complexity and must be configured with the correct power supply. Avoid setting the operating temperatures too high, and maintain suitable running temperature.
Review the Polishing Parameters.
Power supply settings affect performance, so it is important to review the rate of agitation and speed. Keep numbers and units consistent to avoid discrepancies which can cause uneven results or damage.
Troubleshoot Electrode Issues.
Inspect electrodes regularly as small particles or dirt can affect current distribution. Uneven results may occur, so it is recommended to replace or clean old electrodes for optimal results.
Investigate Other Possible Factors.
Check voltage, current, material composition, and temperature. Ensure electrode functionality, and address off parameters for better EP project results.
Applications Of Electrolytic Polishing!
Figure 4 -Electrolytic Polishing Application
Many industries use electrolytic polishing today to achieve desired outputs with it. Let’s explore its applications and practical use.
Metallographic Sample Preparation
Electrolytic polishing has many uses in this field and treats metals for microscopic examination. Modern electrography techniques have advanced to help analytical laboratories create new solutions.
Surface Finishing Of Metal Parts
Electrolytic polishing treats metal surfaces and produces polished, glossy surfaces. Electric current runs through conductive liquids, involving ionic reactions.
An electro-chemical reaction takes place breaking down thin metal surface layers with certain liquid chemicals.
Surface Preparation For Electroplating
Heat treatments and mechanical processes are used to apply decorative or protective plated finishes. But, you may not get consistent results.
Use electrolytic polishing for surface preparation to achieve even coverage and minimal undercut.
Removal Of Surface Defects
Electrochemical cleaning processes are effective for removing surface blemishes like corrosion. No harm is done to materials by the removal of organic and inorganic compounds. In some fields, it removes oxidation particles.
Cleaning And Decontamination
Electrolytic polishing cleans various products which come in contact with foodstuffs or medical equipment.
Electric currents are applied to metal containers or surfaces, removing oils, grease and rust with minimal labor effort.
Weld Cleaning
Welding components require intensive cleaning to remove contaminants before further treatment.
Weld cleaning doesn’t compromise material integrity and removes organic and inorganic compounds from welding points.
Micro-Machining And Micro-Fabrication
Electrolytic polishing is a feasible approach that works with micro-machining components and hollow parts. Precision is key in this plasma electrolytic polishing additive manufacturing branch. Electrolytic polishing metallography and electrolytic polishing aluminum are also used.
Conclusion
Electrolytic polishing refines metals. Many industries achieve mirror–like finishes through techniques and processes, for which KDMFab offers invaluable services.
Electrolytic polishing in dentistry and industrial sector perform well, providing a wide range of solutions.
KDMFab attends to your requirements, and has years of experience accommodating all shapes and sizes to achieve the best possible results. Constant customer service and expertise are provided, making their product unbeatable for polishing needs.
Smooth finishes are in high demand as we are entering the modern age and KDMFab is ideal for electrolytic polishing, helping you to tap its vast potential. Request electrolytic polishing PDF for more information.