Properties of Metalloids Definition
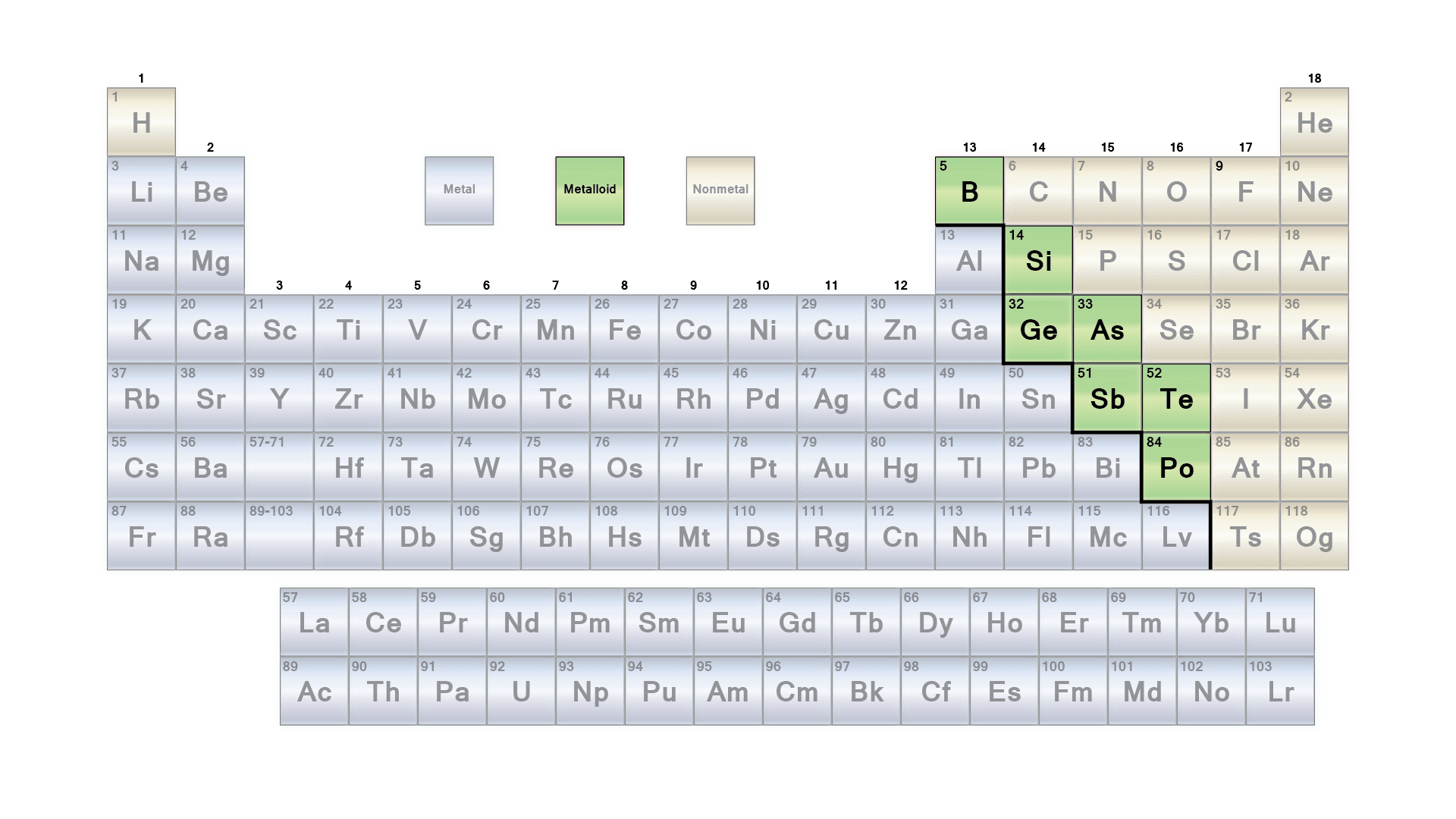
Elements have three classifications. It includes metals, metalloids, and nonmetals. This time, we will talk about metalloids. You can see these elements on the periodic table between non-metals and metals. Commonly known as semi-metals, metalloid is composed of up to class 9 key elements including boron, silicon, germanium, arsenic, antimony, tellurium, astatine, bismuth, and polonium.
Metalloids are elements that exhibit some properties of metals and some of non-metals. It generally looks like metals, brittle, and semiconductors. This article will tackle all the different physical and chemical properties of metalloids. We will also describe which of their property is the most useful.
Scroll down to keep reading and learn more!
Physical Properties of Metalloids
Solid at Room Temperature
All metalloids are found in a solid state at room temperature. Metalloid elements have high melting temperature as can be seen in the table below. (Table 1 shows the melting point of each metalloid element starting from lowest to highest.)
| Elements | Melting Point |
| Tellurium | 449.5°C |
| Antimony | 631°C |
| Arsenic | 817°C |
| Germanium | 938.3°C |
| Silicon | 1410°C |
| Boron | 2079°C |
Appears to Have Metallic Luster
Metalloids typically have metallic luster. Thus, making them look similar to a metal’s physical appearance. In their pure forms, like silicon, can have a metallic luster. For that reason, these types of elements are called metalloids. You can see below the different physical appearances of each metalloid.
They are Brittle
Unlike metals, metalloids are generally brittle and can be broken easily. Thus, some forming techniques used for metals such as cold-forming are not ideally suitable for metalloids. This property limits the metalloid in some applications.
Intermediate Electrical Conductivity
Compared to metals, metalloids have intermediate electrical conductivity. However, these elements are also better electrical conductors compared to nonmetals. These chemistry elements such as silicon are used as a semiconductor. Therefore, making it ideal for the electronic industry.
Density
The density of metalloids can vary. For examples, boron has relatively low density, while tellurium and polonium are denser.
Chemical Properties of Metalloids
Shares Several Characteristics with Non-Metal
There are three ways in which metalloids behaves like non-metals including:
Formation of Anions. Similar to non-metals, metalloids readily gains electrons to become anions.
Covalent Bonds. Metalloids often form covalent bonds when they react with other non-metals.
Multiple Oxidation States. Elements belong to metalloids can vary the number of electrons they gain or lose in chemical reactions. Therefore, creating a compound with different element.
Moderate Ionization Energy
Ionization energy refers to the amount of energy required to remove an electron from an atom or ion. Metalloids usually have ionization energy that is higher than metal but lower than non-metal.
Intermediate Electronegativities
Electronegativity measures an element’s tendency to attract electrons when it forms a chemical bond. Metalloids have more electronegativity than metals but less than the electronegativity of nonmetals.
Known as Amphoteric
Some metalloids like boron are known to have amphoteric behavior. This means they can act as both acids and bases in chemical reactions, depending on the conditions.
Distinguishing and Most Useful Properties of Metalloids
The most distinguishing properties of these elements are that they have a combination of both metal and nonmetal characteristics. Some metalloids like silicon have the ability can act as a semiconductor. This unique feature of metalloids makes them an important element used in the electronic industry. Its semiconducting behavior can be improved using the doping technique.
Identifying Metalloids Through Their Properties
You can identify a metalloid through its physical properties easily just by looking at its appearance. However, it is more difficult to identify metalloids based on their chemical properties since it doesn’t have any characteristics that stand out against metals or non-metals.
Conclusion
In summary, KDMFAB have learned the different physical and chemical properties of metalloids. Metalloids are naturally solid and brittle. It also has a metallic luster, intermediate electrical conductivity, and moderate density when it comes to its physical properties. Metalloids also have intermediate energy ionization, electronegativities, and amphoteric behavior when it comes to its chemical properties.
Frequently Asked Questions
Boron
This is an allotropic element that is very hard. It is also characterized by its strong heat resistance. Its atomic number in the periodic table is 5.
Typically used for making thermal shock-resistant glass along with silicon.
Silicon
This element has a shiny and grayish appearance and is known for its semiconductive properties. It is also characterized by its high boiling and melting points. Its atomic number in the periodic table is 14.
Usually used semiconductors.
Germanium
Germanium is a brittle and hard element. It has an atomic number of 32 in the periodic table.
It is not commonly used as a semiconductor.
Arsenic
Arsenic is a poisonous element characterized by its steel-gray appearance. Its atomic number in the periodic table is 33.
It is commonly utilized for insecticide.
Tellurium
Tellurium has an atomic number 33 in the periodic table. These elements are chalcogen when used together with sulfur and selenium. It is also naturally brittle.
It is typically used as an additive to steel to enhance machinability.
Antimony
Antimony is a brittle and hard metalloid with atomic number 51 in the periodic table. These are usually utilized as a color paints.
Just like other elements, metalloids are also composed of electrons, neutrons, and protons.
| Metals | Metalloids | Nonmetals |
| Solid at room temperature | Solid at room temperature | Solid at room temperature |
| Have a metallic luster | Some have a metallic luster | Dull |
| Malleable and ductile | Brittle | Brittle |
| High electrical conductivity | Intermediate electrical conductivity | Poor electrical conductivity |
| High densities | Moderate densities | Low densities |