With its symbol being Hg, mercury represents the chemical element with the atomic number 80. It will excite you to know that mercury is not just heavy and silvery-white in color. It is also the only metallic element that comes in the form of liquid at normal temperature as well as pressure. It is important for you to know that, mercury is found in the cinnabar or mercuric sulfide form worldwide.
Is Mercury Magnetic?
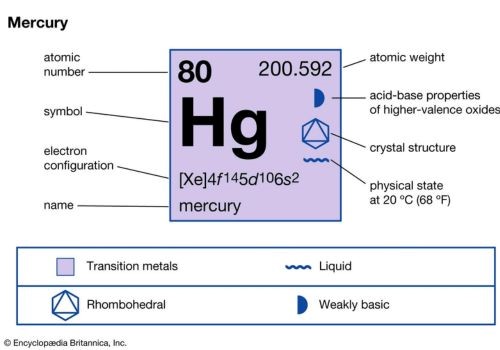
Quicksilver, as mercury is affectionately called, is a unique chemical element. It can be found in water, soil, and the air. So, if you have been wondering if it is magnetic or not, well, although it doesn’t come with a very strong magnetic nature, it has some level of magnetism or magnetic properties. So, yes, it is magnetic.
How Atoms Affect Mercury Magnetism
The atoms of Mercury do not come with complex magnetic features. This means that these atoms do not fall under the pressure of external magnetic fields. Why is that so?
- Mercury atoms come with no unpaired electrons.
- The paired electrons in Mercury’s atoms are what create magnetic moments and make materials magnetic.
Effects of Magnetic Field on Mercury
The magnetic field of mercury is one hundred and fifty times weaker compared to that of the earth. This is due to the fact that, its core cools and solidifies quicker compared to that of the Earth.
Even though the magnetic field of mercury is weaker than that of the earth, mercury is strong to the extent that it can deflect solar wind so as to induce a magnetosphere.
Cage Diffusion Phenomenon in Mercury Magnetism
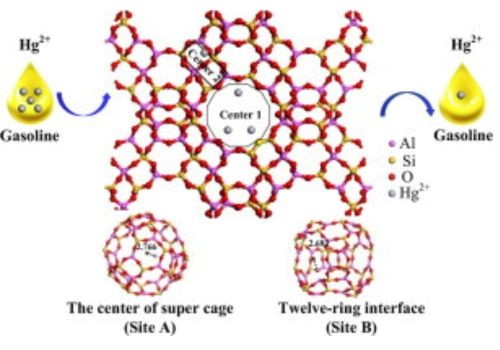
The cage diffusion phenomenon happens when atoms within a liquid get closer to one another. This makes electrons move out of inner, filled shells. This leads to magnetic moments being formed.
It is these magnetic moments that will make mercury repel strongly or weakly against a magnet when external magnetic fields are present.
When all atoms go away from each other, there is a reassembling of their actual shells, which fills them all up and then makes them paired.
Mercury as a Diamagnetic Material
Naturally, mercury is diamagnetic because it has paired electrons within its atomic structure.
How Temperature Affect Mercury Magnetic Properties
The temperature of mecury as an element changes at different temperatures. For instance, it is neither magnetic nor weakly diamagnetic at room temperature.
Is Liquid Mercury Magnetic?
Yes, it is. It is magnetic because magnet poles captivate liquid mercury.
Applications of Mercury due to Non-magnetic Property
- You can find mercury used in barometers, thermometers, blood pressure monitors, or sphygmomanometers.
- You will easily find the mercury cell process, or chlor-alkali, in caustic soda and chlorine production.
- In electrical appliances like mercury vapor lamps, fluorescent lamps, etc., you will find them being used.
- In dental clinics, you will find them used to fill cavities.
- In food manufacturing methods, you will find them used there too.
Conclusion
Knowing the uniqueness of mercury and its magnetic properties will bring you clarity.




