Aluminum’s resistance to both rust allows it to become a multipurpose and long-lasting material for different applications. Learn how aluminum’s unique nature enables it to be adopted across different sectors of the industry.
What is Aluminum?
Aluminum a rare metal, the second most abundant on Earth after oxygen and silicon, but it is incredibly hard to find silicon by itself in nature. It strives to combine with oxygen; this combination forms a protective shield, keeping it glossy and resistant to rusting. It develops a thin oxide layer when oxygenated, leading to further corrosion protection.

Can Aluminum Rust?
No, aluminum does not rust but it does corrode. When oxygen contacts aluminum, it creates a very thin oxide layer on its exterior. This layer works as a protective layer to stop further corrosion.
The corrosion of aluminum is different from rust formation. Rust is a trait exclusive to iron and its alloys in which iron oxides are formed that flake off and leave more metal for oxidation.
On the other hand, the oxide that forms on aluminum is resilient and hugs the surface without letting the corrosion spread deeper.
One way to shield aluminum from corrosion is through the application of coatings or treatments. Such processes include anodizing, which involves thickening the oxide layer via electrolysis, or the use of decorative paints or protective coatings. Through the right maintenance and avoidance of harsh environments, too, the life of aluminum can be stretched out.
Aluminum Corrosion vs. Aluminum Oxidation
Aluminum corrosion stands for a process that is in fact a general degradation of aluminum. The expression ‘oxidation’ is clearer and more accurate, referring to the formation of the oxide layer. To avert the corrosion, you can use protective coatings (like anodizing or paints), along with avoiding flashing aluminum in highly acidic or alkaline environments.

The case of aluminum could make you wonder what separates corrosion and oxidation. However, aluminum does not rust like iron; it just oxidizes when exposed to oxygen. You will notice a white or gray coating on the surface due to oxidation that forms aluminum oxide which stops additional corrosion.
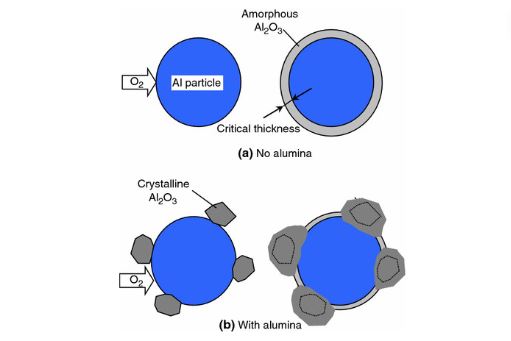
Aluminum Rust and Aluminum Corrosion
One of the questions about aluminum is whether it is prone to rusting and corroding. When it comes to formation of rust on aluminum, it does not happen like in iron. In contrast, it undergoes a process called corrosion, leading to aluminum oxide production when it’s exposed to oxygen. This oxidation layer mainly prevents additional deterioration.
Aluminum can experience different types of corrosion, including:
- Atmospheric Corrosion: This is the most typical corrosion that looks like a coating of metallic oxide, which is a result of exposure to the atmosphere. This leads to a white, flaky cover for that aluminum.
- Galvanic corrosion: This type of corrosion develops when aluminum is next to a more noble metal, for instance, copper or steel. The aluminum will get damaged due to corrosion at a higher rate than the one made of metal.
- Pitting corrosion: This kind of precise hole positioning is attributed to a specific corrosion form called the asporic pores. It is generally attributed to the presence of salt, which would be found in salt spray.
- Intergranular corrosion: These corrosions attack along the grain boundaries in the case of aluminum. It can decimate metallic structures and lead to their collapsing.
- Exfoliation corrosion: This kind of corrosion causes the top layer of the aluminum to peel off. It is often the result of high temperatures and pressures in the environment.
- General corrosion: This kind of corrosion is a uniform attack on the aluminum surface.
- Deposition corrosion: This kind of corrosion is attributable to the depositing of the corrosive material on the aluminum surface.
- Stress corrosion cracking (SCC): In this case, the corrosion is the result of the synergistic action of both stress and corrosion. It can cause sudden and unexpected failures.
- Erosion corrosion: This type of corrosion is the result of the removal of the aluminum oxide layer by the action of sand, wind, or water.
- Corrosion fatigue: This type of corrosion is a product of the fast cyclic loading of the aluminum.
- Filiform (or wormtrack) corrosion: In this kind of attack, small, thin fibers come out on the surface of the aluminum. It is commonly caused by contact with chlorides and organic substances.
- Microbiologically induced corrosion (MIC): This corrosion is a consequence of the microbiological activity.

Reasons why Aluminum Does Not Rust.
Aluminum is corrosion-resistant due to its inherent rust-resisting properties. The protection of aluminum oxide layer formation and the chemical behavior of aluminum are the main reasons for its good resistance to rust. Aluminum starts to form an aluminum oxide film on its surface, which tightly sticks to the metal when oxygen gets in contact.
This oxide layer makes up a shield that stops any further oxidation and corrosion. Furthermore, aluminum possesses the distinctive property of not oxidizing because it does not contain iron, which aids in rust formation when present. Unlike iron-based metals, which are predominantly composed of iron elements, aluminum uses the same valence, i.e., aluminum.
Furthermore, aluminum’s oxide layer has a stable and self-healing character. Despite the surface being scratched or damaged, the oxide layer re-forms quickly, which ensures that corrosion protection remains in place.
Uses of Aluminum Due to Rust-Resistant Properties
Building and Construction: Aluminum’s strength, formability, and sturdiness against corrosion are the main reasons it is preferred by builders. The material is employed for roofing, siding, window frames, and building facades.
Transportation: The fact that this material is lightweight and is not susceptible to corrosion makes it very popular in the transportation sector. Aluminum is used in airplanes, ships, cars, and trucks due to its low weight and excellent fuel efficiency.
Food Packaging: The lightness, malleability, and non-rusting properties make aluminum foil and containers popular for food packaging. This way, the produce is well protected from cold or tampering.
Consumer Electronics: Aluminum is kind of a heavyweight metal in the electronics industry because it is very effective at tolerating heat and is not susceptible to corrosion. See the image of it on the screens of your lap-tops, smartphones, or other gadget devices.
Electrical Wiring and Components: Aluminum’s rust resistance is an ongoing trend for electrical wiring, and the efficacy of the efficacy of the components is commonly that of choice. It maintains reliability and safety in the operation of electrical equipment, like those working in an outdoor environment or in humid surroundings.
Heat Exchangers: Aluminum is impressive in that it can resist corrosion and conduct heat efficiently. Therefore, it is widely used in manufacturing heat exchangers that are used in HVAC systems, refrigeration units, and automotive radiators.
Aerospace Industry: Aluminum alloys are preferred in aerospace engineering because of their low weight and corrosion resistance. Aircraft parts, like fuselages, wings, and jets, thank aluminum for keeping the structure strong in extreme situations.
Marine Applications: Aluminum is very popular in the marine ecosystem for boat hulls, shipbuilding, and offshore structures. Its ability to resist oxydation permits it to last longer in a salty environment without falling prey to corrosion.
Automotive Industry: Aluminum is a material that is widely used in the automotive sector for making engine blocks, wheels, and body panels. Its ability to stand up to road salts and moisture extends the service life of the vehicles and improves their fuel economy because of their lower weight.
FAQs
What Does Aluminum Corrosion Look Like?
You can notice corrosion on aluminum through a gray or white mass on its surface. As the damage worsens, pitting or etching may be observed on the metal surface.
How Long Will It Take Aluminum to Rust?
While aluminum doesn’t rust like iron, there is a possibility of it corroding when certain conditions are present. The corrosion speed can be affected by elements like environmental exposure, moisture level, and the existence of corrosive agents.
Will Aluminum Rust If It Gets Wet?
Aluminum tends to corrode and not rust when it is in contact with water and oxygen, causing the formation of aluminum oxide. In any case, however, the oxide layer usually prevents aluminum from rusting.
What Causes Aluminum To Corrode?
Aluminum corrosion is mainly due to the pollution of moisture and oxygen, which starts the development of aluminum oxide. Factors like exposure to salt, acid, or alkaline substances can also promote the corrosion process.
Does Aluminum Rust In Saltwater?
No, aluminum oxidizes at a faster rate in saltwater due to the presence of chloride ions, which can destroy the protective layer of oxide faster. Proper corrosion-resistant coatings or treatments should be applied to aluminum for saltwater applications.
Does Cast Aluminum Rust?
Unlike other aluminum alloys, cast aluminum may rust, but the level of corrosion depends on the specific alloy composition and surface treatment. There should be continuous maintenance of cleanliness, surface protection, and buffer coatings to reduce corrosion in cast aluminum.
Does Anodized A Rust?
Yes, but not in a destructive way. One of the key advantages of the anodic oxidation of aluminum is the durability of the resulting aluminum oxide, which is over 10 times thicker and stronger than the natural oxide. This barrier additionally improves the lifespan and corrosion immunity of the aluminum surface.
Will Aluminum Alloy Rust?
Aluminum alloys, unlike pure aluminum, do not rust. Conversely, they can be eroded under special conditions, which are subject to the alloy mixture and atmospheric exposure. The right type of alloy or surface treatment could mean better corrosion resistance for aluminum alloys.
Does Aluminum Rust Faster than Steel?
Aluminum oxidation happens faster than that of steel.
Does Aluminum Rust in Chlorine?
Aluminum alloys suffer measurable general corrosion and localized corrosion in raw and chlorinated raw water.
Does Aluminum Rust in Shower?
Aluminum is a great bathroom material that does not rust.
Does Aluminum Rust in Rain?
Aluminium does not rust or corrode in moist conditions.
Conclusion
Wherever it is in aerospace, marine, automotive, construction, or household items, aluminum’s rust resistance properties offer you reliability and sustainability. By allowing the properties of aluminum to be fully utilized and implementing standard anticorrosion solutions, industries will be able to continue drawing positive.
More Resources:
Aluminum Melting Point – Source: KMDFAB
Density of Aluminum – Source: KDMFAB
Aluminum Alloys – Source: KDMFAB
Aluminum Corrosion – Source: SCIENCE DIRECT




