Diving into the density of aluminum is like going on an amazing treasure hunt. In this blog, you will learn tons about how snug aluminum atoms are. Plus, you will become a smart cookie about units like kg/m^3. You’ll explore why knowing aluminum’s density is a big deal.
Understanding Density!

Pic 1
Definition of Density
Density tells how much stuff fits in a space. For aluminum, density measures how heavy a piece is for its size. Aluminum’s density is around 2.7 grams per cubic centimeter (g/cm³).
Another way to talk about the density of aluminum in g/cm3 is 2,700 kilograms per cubic meter (kg/m³). So, a cube of aluminum that’s one meter on each side weighs 2,700 kilograms.
Units of Measurement for Density
- kg/m^3
- g/cm^3
- lb/ft^3
- lb/in^3
- oz/in^3
- slugs/ft^3
- ton/yd^3
- Atomic units
- Planck units
- Imperial units
- Troy units
- SI units
- CGS units
Density of Aluminum!

Pic 2
Standard Density of Aluminum
The density of aluminum is how much stuff is packed in a space. Pure aluminum has a density of 2.7 grams per cubic centimeter (g/cm3). A cubic foot of aluminum weighs 168.5 pounds.
Aluminum is light! In fact, the accepted value for density of aluminum is 2,700 kilograms per cubic meter (kg/m3). Compare this with gold. Gold is way heavier. A cubic foot of gold weighs 1,206 pounds. Airplanes and soda cans use aluminum. Aluminum keeps them light and strong.
Factors Affecting the Density of Aluminum
· Alloy composition
Aluminum loves to mix with other metals. When mixed, the duo becomes an alloy. For example, density of aluminum 6061 lb/in3 is 0.0975. The 6061 alloy is made up of aluminum, magnesium, and silicon. These buddies help make it strong.
So, aluminum 6061 is great for making bikes and parts of planes. It’s tough and light.
·Temperature
As the heat goes up, aluminum gets bigger but lighter. Imagine a balloon. When you blow air into it, the balloon gets big and light. Similarly, at 20°C, aluminum’s density is 2.70 g/cm3. But if you heat it to 500°C, the density goes down to 2.57 g/cm3. So, when you deal with aluminum, keep an eye on the temperature.
·Impurities
Sometimes, tiny bits of other stuff get in aluminum. These bits are impurities. They can change how heavy or light aluminum is. Impurities make aluminum’s density different from the pure stuff. For instance, let’s talk about aluminum foil.
You might ask, “what is the density of aluminum foil?” Aluminum foil is not pure. It has impurities. The density of aluminum foil is a bit different from pure aluminum.
But, still it’s close to 2.7 g/cm3. So, when you use aluminum, check if it’s pure or has impurities. Pure and impure aluminum may not always act the same.
·Mechanical processing
Mechanical processing involves changing aluminum’s shape. When rolling and extruding aluminum, what is the density of aluminum in g ml alters.
As an expert, the density changes from 2.70 g/cm3. For instance, in density of aluminum 6061 lb in3, hammering or pressing influences 0.0975 lb/in3 values. Consequently, Aluminum’s resistance to scratching heightens.
·Grain size
Aluminum’s grain size dictates density of aluminum lb in3. Large grains mean lower density, like 0.0975 lb/in3. Conversely, small grains equal higher density.
Moreover, density of aluminum 5052 depends on grain size. Specifically, 5052 alloy, utilized in ships, has a value around 0.097 lb/in3. So, small grains in 5052 aluminum ensure robustness for marine vessels.
·Phase structure
Atoms in aluminum arrange themselves in patterns. Sometimes, patterns are random. In other cases, patterns are orderly. Now, density of aluminum g cm3 shifts with the phase structure. For theoretical density of aluminum, assuming perfect arrangements, values approach 2.70 g/cm3. However, real-life aluminum doesn’t achieve perfect patterns, affecting density values.
·Heat treatment
Dwell on aluminum heat treatment. Aluminum changes when heated or cooled. For instance, annealing involves heating and slowly cooling. This makes aluminum softer. Density of aluminum imperial units might slightly vary.
Quenching, on the other hand, involves fast cooling. So, 6063 alloy, often in window frames, gets tougher with quenching. Understanding heat treatment is critical for manipulating aluminum density.
·Pressure
Another important factor is pressure. Compressing aluminum increases the density of aluminum is 2.70 g cm3. By compacting atoms, density escalates.
Furthermore, applying pressure ensures stronger bonds among atoms. For example, pressure treatment raises density in 7075 aluminum, predominantly used in aerospace.
Manufacturers opt for high-pressure techniques to fulfill specific application requirements.
·Cooling rate
Rapidly cooling aluminum results in smaller grains hence increased density. Contrastingly, slow cooling leads to larger grains, and therefore lower density.
Aluminum alloys, such as 2024 used in aircraft structures, demand precise cooling rates.
Consequently, maintaining exact cooling speeds ensures optimal performance and safety in aviation applications. Experts meticulously control cooling rates for ideal density properties.
·Precipitation Hardening
Precipitation hardening changes the density of aluminum is 2.7 g cm3. Small bits called precipitates form in the metal. The metal then becomes hard and strong. Aluminum alloys like 6061 use this trick. First, heat the aluminum. Then, cool it fast. Now, let it rest.
·Work Hardening
When aluminum gets hammered or rolled, something amazing happens. Aluminum has a density of 2.70 g cm3, but work hardening can tweak this.
The atoms inside the aluminum move around. The metal becomes stronger and harder. No heat needed! However, too much hammering can make it brittle.
·Aging
Aging is like a superhero power for aluminum. During aging, the density of solid aluminum is 2.70 and it gets even stronger. Aluminum waits in a cozy spot.
The atoms inside it make a neat pattern. Tiny, strong parts called clusters form. That’s natural aging. There’s also artificial aging – heating it up to speed things up!
·Deformation History
How aluminum got its shape matters. Pressed, rolled, or hammered – each tells a story. For example, density of aluminum kg m 3 is 2,700.
Yet, a rolled sheet may differ slightly in density. Rolled sheets are thinner and can be used for cans. Pressed aluminum makes great foils. Each process crafts aluminum’s density tale.
·Surface Coatings
Aluminum loves to play dress-up with coatings. Sometimes aluminum has a density of 2.70 g/cm3, but then a coating hops on. Aluminum powder coatings, paint, or anodizing can change the game. The density changes a little. Coatings protect aluminum from scratches and rust.
·Hydration State
What’s the density of aluminum when water joins the party? When aluminum gets wet, it forms aluminum hydroxide. There’s a small change in density. The density of aluminum foil in g cm3 gets affected by hydration. Wet aluminum gets bigger and lighter.
Comparison with Other Metals’ Densities
| Metal | Density (g/cm³) | Atomic Number | Melting Point (°C) | Boiling Point (°C) | Atomic Radius (pm) | Crystal Structure |
| Iron (Fe) | 7.874 | 26 | 1538 | 2862 | 126 | Body-centered cubic |
| Gold (Au) | 19.32 | 79 | 1064 | 2970 | 135 | Face-centered cubic |
| Copper (Cu) | 8.96 | 29 | 1085 | 2562 | 128 | Face-centered cubic |
| Titanium (Ti) | 4.506 | 22 | 1668 | 3287 | 147 | Hexagonal close-packed |
| Nickel (Ni) | 8.908 | 28 | 1455 | 2913 | 124 | Face-centered cubic |
| Tungsten (W) | 19.25 | 74 | 3422 | 5555 | 137 | Body-centered cubic |
| Platinum (Pt) | 21.45 | 78 | 1768 | 3825 | 139 | Face-centered cubic |
The table on Comparison with Other Metals’ Densities!
Density Variations Among Aluminum Grades!
| Property/Terms | 1100 Series | 2024 Grade | 3003 Grade | 5052 Grade | 6061 Grade | 7075 Grade |
| Density (g/cm³) | 2.71 | 2.78 | 2.73 | 2.68 | 2.70 | 2.81 |
| Primary Use | General purpose, high-thermal conductivity | Aerospace structures, fasteners | General fabrication, pressure vessels | Marine applications, signage | Structural applications, aircraft, bridges | Aircraft, high-strength applications |
| Aluminum Content (%) | >99 | 90.7-94.7 | 98.6 | 95.8 | 95.8-98.6 | 87.1-91.4 |
| Typical Alloying Elements | 0.12 Cu (copper) | 4.3-5.0 Cu, 0.3-0.9 Mn, 1.2-1.8 Cr | 1.0-1.5 Mn | 2.2-2.8 Mg, 0.1-0.4 Cr | 0.8-1.2 Mg, 0.4-0.8 Si | 5.1-6.1 Zn, 2.1-2.9 Mg, 1.2-2.0 Cr |
| Weldability | Excellent | Poor | Good | Very Good | Good | Poor |
| Strength (MPa) | 90 | 469 | 145-200 | 214-262 | 310 | 572 |
| Corrosion Resistance | Good | Poor | Good | Excellent | Good | Average |
Table on Density Variations Among Aluminum Grades!
Common Aluminum Alloys and Their Compositions!
·6061-T6
The density of aluminum lb in 3 is 0.0975 for this alloy.
·7075-T6
A density of aluminum g ml of 2.81.
·2024-T3
Density of aluminum lb/in3 of 0.101.
·3003-H14
A density of aluminum g/ml of 2.73.
·5052-H32
A density of 5052 aluminum standing at 2.68 g/cm3.
·1100-H14
2,700 kilograms per cubic meter
·5083-H32
Its density is a smidge higher at 2,660 kilograms per cubic meter.
·Al-Li alloys
They boast a lighter density of about 2,600 kilograms per cubic meter. A mix of aluminum and lithium, this alloy makes planes and spaceships. Lightness and strength are its two big wins.
·Al-Si alloys
A density close to 2,680 kilograms per cubic meter.
·Al-Cu alloys
Al-Cu alloys stand tall with a density of around 2,800 kilograms per cubic meter.
·Al-Mg alloys
A density of 2,650 kilograms per cubic meter.
·Al-Zn alloys
The density of Al-Zn alloys is 2,780 kg/m³.
·Al-Fe alloys
Al-Fe is heavy. 2,900 kg/m³ is the density. Cars and trains need Al-Fe.
·Al-Ni alloys
The density is 3,000 kg/m³.
·Al-Sc alloys
The density is 2,850 kg/m³.
Density Calculations and Practical Applications!

Pic 3
How to Calculate the Density of Aluminum?
·Mass measurement
In labs, precise scales measure the mass density of aluminum. Normally, the value hovers around 2.7 grams per cubic centimeter (g/cm³). For instance, the density of 6061 aluminum, a well-regarded alloy, is similar. Next, consider density of pure aluminum.
Surprisingly, the number is almost the same. Thus, a small sample of aluminum weighing 50 grams takes little space, underlining the metal’s practical use in lightweight structures.
·Volume measurement
Free electron density of aluminum, when measuring the volume, immersion in liquid offers precise outcomes. Now, understanding electron density of aluminum proves essential.
Significantly, 18 x 10²³ electrons per cubic meter concentrate in aluminum.
Furthermore, the electrons’ arrangement helps in creating lightweight, durable materials. So, a cube with sides measuring 2 cm boasts a volume of 8 cubic centimeters (cm³). Such a cube exhibits the usefulness of aluminum’s volume in manufacturing.
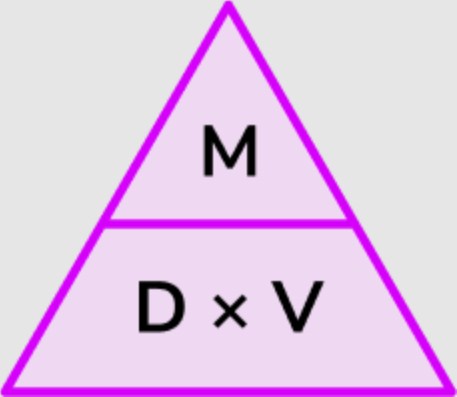
·Density formula
What is the density of aluminum formula? To find out, apply this formula: density = mass/volume. For example, if 40 grams of aluminum occupies 15 cubic centimeters, divide 40 by 15. Remarkably, the answer is approximately 2.7 g/cm³, similar to the known density. The formula demonstrates a reliable method for determining aluminum’s density, facilitating the development of high-performance materials.
·Unit conversion
Diverse industries utilize varied units. For instance, American industries prefer using density of aluminum lb/ft3, which is around 168 lbs per cubic foot. Alternatively, others opt for density of aluminum lb ft3, with no difference in values.
Converting 2.7 g/cm³ to lbs/ft³, you’ll get the same 168. With uniform conversions, engineers across the globe can communicate effortlessly, fostering innovation in aluminum applications.
·Archimedes’ Principle
To find the density of aluminum, employ Archimedes’ principle. Firstly, take a piece of aluminum. Note down its mass in grams. Next, get a container full of water. Let the aluminum dangle in the water without touching the bottom.
Observe how much water rises. That’s the aluminum’s volume. Now, divide mass by volume. Aluminum’s density is approximately 2.7 grams per cubic centimeter.
·Hydrostatic Weighing
Another superb method is hydrostatic weighing. Secure a piece of aluminum and a scale. Submerge the aluminum entirely in water. Check the scale’s reading. Subtract this number from the weight in air. The difference is the buoyant force.
Then, divide the aluminum’s weight in air by this buoyant force. Generally, the result is close to 2.7 g/cm^3, which is aluminum’s density. Engineers favor this method for density calculations.
·Pycnometer Method
Pycnometer, a sealed flask, is key here. Record the pycnometer’s weight, and then fill it with a known volume of water. Again, jot down the weight. Now, replace the water with aluminum and re-weigh. Subtract the first weight from the last.
Divide by water’s density (1 g/cm^3). Finally, divide aluminum’s mass by the number obtained. A result near 2.7 g/cm^3 indicates success. Laboratories across the globe value this method for precision.
·Gas Displacement
In gas displacement, employ helium or nitrogen. Place aluminum in a chamber, and then purge the air. Fill with gas. Monitor the pressure difference using a highly sensitive transducer. Extract the volume from these readings.
How to find density of aluminum, divide aluminum’s mass by volume to find density. Cutting-edge manufacturers adopt gas displacement for its reliability in evaluating aluminum’s density and porosity.
·X-ray Crystallography
A high-tech approach, X-ray crystallography, is also in play. Direct X-rays at a sample of aluminum. Analyze the diffraction pattern on a detector.
By solving complex equations, extract atomic structure data. Use Avogadro’s number, atomic weight, and unit cell volume to calculate density.
The result should align with the accepted 2.7 g/cm^3. World-renowned scientists embrace X-ray crystallography for probing atomic structures and determining densities with unparalleled detail.
·Ultrasonic testing
A smart method, ultrasonic testing measures the density of aluminum. Sound waves move through aluminum quickly. Remarkably, at 6,320 meters per second! To find density, divide aluminum’s mass by its volume.
Experts favor this method due to its reliability. Additionally, ultrasonic testing safeguards materials from damage. Many industries count on ultrasonic testing for top-quality aluminum.
·Digital density meter
A nifty tool, the digital density meter, offers precise data. Here’s a secret: pure aluminum has a density of 2.7 grams per cubic centimeter. This meter’s probes dive into aluminum. Subsequently, the device reads the density.
Engineers and scientists stand by these numbers. The digital density meter speeds up the process. On top of that, better decisions are made with this information.
·Temperature correction
Aluminum changes when it gets hot or cold. As a fact, for every Celsius rise, aluminum expands 0.000022 times its size. This means its density drops! So, experts fix the numbers to match normal room temperature, 20°C.
This adjustment ensures accuracy. In turn, engineers craft impeccable products. Without temperature correction, cars, computers, and wires wouldn’t be as dependable.
·Pressure correction
Pressure impacts aluminum, too. Experts know high pressure packs aluminum particles closer. So, the density goes up. In outer space, pressure plummets.
Consequently, aluminum’s density dips. With pressure correction, aluminum in rockets and satellites can withstand space’s harsh conditions.
The data is tuned to Earth’s atmospheric pressure, 101.325 kilopascals.
·Material purity
Material purity affects aluminum’s density. Pure aluminum boasts 2.7 grams per cubic centimeter density. Sometimes, small particles sneak into the mix. Consequently, experts test for impurities. Then, they calculate the accurate density.
·Alloy composition
Mix aluminum with other metals and get an alloy. Changing the alloy’s recipe alters aluminum’s density. For instance, aluminum with copper is denser than with magnesium. Experts examine alloy composition closely.
Furthermore, they calculate density for each blend. Vehicles, cans, and machinery benefit from these alloys. Each aluminum alloy serves unique purposes.
Alloy composition dictates aluminum’s destiny. Therein, the knowledge of alloy composition proves indispensable.
Practical Applications of Density Calculations
 Pic 4
Pic 4
·Material selection
Engineers love aluminum. The average density of aluminum is 2.7 grams per cubic centimeter. In contrast, steel packs a hefty 7.8 grams in the same space. Knowing the density of aluminum vs steel helps in picking materials for cars and planes.
Aluminum makes objects lighter. So, airplanes made with aluminum fly higher and cars use less gas. Numbers and stats guide smart choices.
·Structural analysis
In building large structures, experts use aluminum for strength and lightness.
Especially, like the density of 6061-t6 aluminum and the density of 6061-t6 aluminum, which is the same thing said differently. Also, the density of 7075 aluminum, at 2.8 grams per cubic centimeter, is a favorite. Engineers know the known density of aluminum to make buildings that won’t fall over.
·Fluid dynamics
Aluminum oxide is a cool material. The density of aluminum oxide is around 3.95 to 4.1 grams per cubic centimeter. That’s more than aluminum itself. In fluid dynamics, scientists study how liquids and gases move around objects.
Aluminum oxide has special ways of interacting with fluids. So, aluminum oxide gets used in high-tech stuff like rocket parts.
·Thermal management
Heat is tricky. Some metals are better at handling heat than others. Aluminum is one of them. The density of aluminum at 25°C is about 2.7 grams per cubic centimeter.
With the accepted density of aluminum, experts can create computer parts and car engines that won’t get too hot. They use aluminum to make sure the heat moves away from the important parts.
·Packaging design
Aluminum, with a density of 2.7 grams per cubic centimeter, proves vital in packaging design. In soda cans, for instance, the metal’s lightness offers fuel efficiency during transport. Furthermore, aluminum forms a barrier against light, oxygen, and bacteria.
Food remains fresh. The aluminum foil, a mere 0.016 mm thick, acts as a shield.
·Acoustics
In acoustics, aluminum density presents unique traits. Because sound travels through aluminum at 6,420 meters per second, designers prefer it in high-fidelity speakers. Speakers typically have diaphragms made of aluminum.
Moreover, the density offers distinct acoustic impedance, ensuring superior sound quality. An aluminum alloy, 6061, provides added stiffness. So, many studios and audiophiles prize aluminum for sonic performance.
·Material handling
Material handling systems, such as conveyor belts, often incorporate aluminum. A typical aluminum beam, 6063 alloy, can support substantial loads due to its high strength-to-weight ratio.
Aluminum pallets, known for being light yet robust, ensure easy handling and transportation. Moreover, aluminum does not spark, making handling processes in explosive environments secure. Here, material handlers count on aluminum’s density for efficiency and safety.
·Process optimization
Manufacturing industries employ aluminum to optimize processes. With a melting point of 660.3°C, aluminum gets cast into shapes swiftly.
Alloys like 7075 are prominent for being hard yet lightweight. The density-related properties of aluminum, such as thermal conductivity, facilitate quicker cooling. Consequently, process optimization benefits from the metal’s attributes.
·Inventory management
Inventory managers value aluminum’s density for storage solutions. With its lightness, stacking aluminum products, even at considerable heights, remains feasible.
Aluminum’s corrosion resistance is another asset, which means long shelf lives for goods. By calculating the metal’s density, inventory specialists maximize space utilization. For example, a rack with an aluminum frame can sustain substantial weights.
Ergo, inventory management with aluminum enhances both space and resource optimization.
·Recycling processes
In recycling, the density of aluminum proves vital. Aluminum boasts a density of 2.7 grams per cubic centimeter. Comparatively, steel has 7.8. Ergo, aluminum’s lightness is a boon for recycling plants. These plants handle thousands of kilograms daily.
They separate metals using density-based machines like air classifiers and eddy current separators. Moreover, lower energy is necessary for melting aluminum than denser metals.
·Cost analysis
In manufacturing, the density of aluminum impacts expenses. Aluminum sheets and blocks cost less due to lower density. Additionally, the raw material weight is essential for shipment and production.
Lighter materials such as aluminum have reduced transportation costs. By selecting aluminum over steel, manufacturers save on fuel and shipping. Plus, the lower density means that the material is easier to mold, reducing processing costs.
·Sustainability assessments
Grasping aluminum’s density is indispensable for sustainability assessments. The low density signifies lesser raw material and energy in production.
Consequently, the carbon footprint dwindles. The annual global production of aluminum is 63.2 million metric tons. The low density of aluminum results in reduced greenhouse gas emissions compared to denser metals. Moreover, aluminum’s recycling ability further mitigates environmental repercussions.
·Life cycle analysis
In Life Cycle Analysis, aluminum’s density plays an instrumental role. From mining bauxite to producing end products, each phase is under scrutiny. Aluminum’s density, 2,700 kg/m³, influences resource utilization.
Lighter than other metals, aluminum warrants reduced energy and resources throughout the product’s life. Furthermore, the durability and corrosion resistance due to its low density enhance the product lifespan.
·Waste reduction
Embracing aluminum is a cogent move to curtail waste. As aluminum is lightweight due to its low density, lesser material is necessary for production. Thereby, industrial processes yield minimal scrap.
Moreover, aluminum’s high recycling rate. Society reaps the benefits with lower pollution and landfill masses. Indeed, aluminum’s density is a cornerstone of sustainable and waste-reducing practices.
Conclusion
In a nutshell, aluminum’s density tells how close atoms are to each other. You’ve taken a deep dive into all sorts of units like lb/ft^3.
Figuring out the density is like solving an amazing puzzle. The numbers help in doing so many cool things with aluminum. Now, put this powerful knowledge to use! Visit KDMFAB to see how aluminum rules the world of materials.




