The only non-metal material that can conduct electricity is graphite. While other metals show certain melting points, graphite, on the other hand, has complex and high melting points.
The Melting Point of Graphite is:
- 3650 degrees Celsius
- 6602 degrees Fahrenheit.
Composition of Graphite
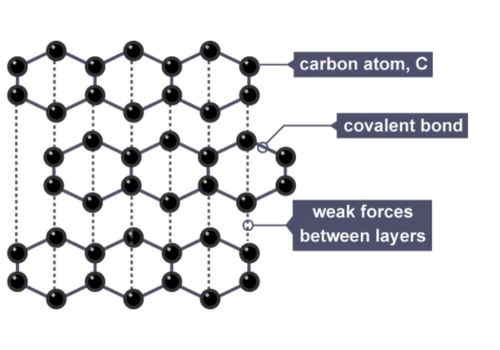
Graphite is one of the allotropes of carbon, which means they both have the same elements but different arrangements of atoms.
Likewise, carbon atoms in diamond makes a rigid 3D network. On the other hand, Graphite has a stacked layer of carbon atoms which are all connected to 3 other carbon atoms via sp2 hybridization forming a hexagonal ring with 3 long covalent bonds and delocalized electrons.
Meanwhile, the weak connection between these layers lets them move easily, which makes them slippery and a good conductor of electricity and heat.
| Property | Value |
| Crystal Structure | Layered hexagonal |
| Color | Black, Grayish black |
| Density | 2.2 g/cm³ |
| Melting Point | Sublimes at ~3650 °C (6582 °F) [1] |
It happens when the solid directly changes into gas skipping being liquid.
Research Trends in Graphite Melting Behavior
With the growing field of science and technology, researchers are continuously studying the melting behavior of graphite. As you know, the more the temperature rises, the more it can affect the surrounding environment.
Meanwhile, graphite experts are looking for a way to understand how much pressure they can create so that graphite can turn into liquid.
There is an advanced computer system that provides details about the atomic-level behavior of graphite under extreme temperatures. Meanwhile, this can increase our understanding of the melting point of graphite. This will help in the future about the nature of graphite and find possibilities to reduce its impact on the environment.
Factors Influencing the Graphite Melting Point
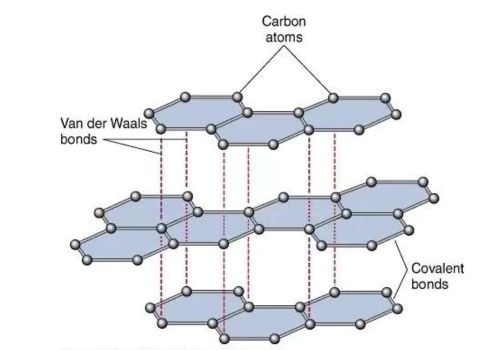
Graphite, with its layered structure, influences phenomena called sublimation instead of melting. Meanwhile, this sublimation process only happens when the solid graphite converts directly to gas with a high temperature of around 3650°C. There are certain factors that can affect the Graphite melting point as well.
Meanwhile, there are weak Van der Waals forces between the layers of graphite that need less energy to overcome as compared to the strong covalent bonds inside these layers.
Likewise, this phenomenon allows the layer of graphite to readily transition from solid to gas phase at high temperatures. So, the outcome is only high pressure can increase the melting point of graphite.
However, when somehow you apply strong pressure on graphite, it pushes the spaces between the layers, and it makes it more difficult for the layers to separate and enhance the sublimation process.
Applications of Graphite in High-Temperature Environments
The Unique characteristics of graphite make it ideal for different high-temperature uses, from refractory linings, such as graphite crucibles, to furnace lining.
Meanwhile, it can handle extremely high temperatures, which is perfect when you want to melt metals.
Additionally, graphite has good heat conductivity and is very valuable for high-temperature electronics. Likewise, it is used as a heat sink and other heat-dissipating components.
The interesting use of graphite is in rockets to resist extreme heat when the rocket propulsion happens.
Conclusion
No doubt, graphite melts at high temperatures, which is never easy to achieve and can impact badly on the environment. The use of graphite in science, industry, and daily life makes graphite different from all other substances, including sublimation at such high temperatures.
More resources:
Carbon Graphite – Source: KDM
What is Graphite -Source: East Carbon
Aluminum Melting Point – Source: KDM
Copper Melting Point – Source: KDM
Melting Point of Zinc – Source: KDM
Melting Point of Magnesium – Source: KDM




