Understanding the melting point of copper is critical before choosing this metal for any application. It helps you know the limits within which copper metal will not lose its integrity or transform into a liquid state.
In this guide, we will explore all the fundamental facts about copper metal melting point. From what it is, factors affecting the melting point, and other heat or temperature characteristics.
Let’s dive right:
What is the Melting Point of Copper?
Copper melting point, also referred to as the melting temperature of copper is an equilibrium point where this metal virtually exists in two phases (liquid and solid phase).
That is, it is a temperature beyond which copper metal transitions from solid to liquid phase. Of course, during this transition, there are many structural and chemical changes in the copper material.
Whenever you are analyzing the temperature at which copper melts, it is critical to be specific. Remember, we have pure copper and copper alloy.
Again, the unit of measurement is also a critical aspect. We shall explore more details on the melting point of copper and units of measurements shortly.


Why Know the Copper Melting Points
There are many reasons why knowing when copper melts is critical:
- Helps copper fabricating technicians determine whether the material is suitable for their project or not.
- Reduces chances of copper structural failure due to high temperature exceeding the melting point
- Knowledge helps in choosing the right furnace and energy source for copper fabrication and heat treatment
Remember, in most structural applications failures such as creep will begin to occur as copper approaches the melting point. Therefore, knowing the melting temperature of copper will help you operate the structure within safe temperatures.
Melting Point of Pure Copper
Ideally, this refers to copper without any added additives or elements. Ideally, pure copper begins to melt at:
| Material | Melting Point of Copper in Celsius (°C) | Melting Point of Copper in Fahrenheit (°F) | Melting Point of Copper in Kelvin (K) |
| Pure Copper (Cu) | 1,085 | 1,984 | 1,357 |
Ideally, we define the melting point of metals based on temperature. Remember, melting copper requires heat, which is essentially based on the level of temperature.
Although there could be many forms in which copper exists such as nanoparticles, mesh, foil, powder, tubes, rods, wire, or sheet, the melting remains within the above range.
At times you may find the melting temperature of copper defined in other measurement units such as Rankine, electron volts, joules per mole, or BTUs per pound. Well, all these are derived from the above temperature units.
Melting Point of Copper Alloys
For most engineering applications, copper alloys have superior properties. A reason, we rarely use copper in its pure form.
Now, every element – whether a non-metal or metal has unique melting points. What this implies is:
Whenever you add any element to pure copper, expect the melting temperature to change. Usually, the melting point will be a reflection of the overall alloy composition. However, the combination of elements you can add to copper is unlimited.
For the scope of this guide, we will focus on the melting point of copper alloys that are commonly used in engineering applications.
Let’s simplify these metal melting points in the table below:
| Copper Alloy | Melting Temperature of Copper Alloys (°C) |
| Arsenic copper | 685 |
| Beryllium copper | 870 to 980 |
| Brass | 930 to 940 |
| Bronze | 913 |
| Cupronickel | 1170 to 1240 |
| Chinese silver | 961 |
| Gunmetal | 900 to 1000 |
Now, you can compare the values above with the temperature copper melts – 1,085°C. You can see the variation in the melting temperature of copper alloys.
Atmospheric Pressure Vs. Copper Melting Temperature
At times, you may wonder why the melting point of pure copper or its alloys may fluctuate. Well, this is due to certain environmental factors such as atmospheric pressure.
In most cases, the melting point of metals increases with an increase in pressure. The same applies to the melting temperature of copper.
Therefore, the melting point of copper will vary depending on environmental conditions. Usually, when processing copper, you can choose certain special conditions. It will help save on the high energy cost of melting copper.
Let’s look at some practical scenarios and their effect on the copper melting temperature range:
- Ambient pressure – pure copper melting point is 1,085°C. However, the melting point of Cu will reduce as you move to higher altitudes such as the mountain top.
- High pressure – it requires more heat to melt copper, hence a high melting point than in ambient air.
Therefore, when evaluating copper melting point, you must consider the environment.
Impurity in Copper
Generally, impurities lower the melting point of metals, so copper’s melting temperature is not an exception. On the other hand, the same impurities are likely to increase the boiling point of copper.
Reason – impurities tend to stabilize the liquid phase of copper.
That is to say, the melting point of impure copper is relatively low. Remember, the alloying elements can form part of “impurity”. So, you can add an alloying element to improve the desirable properties of copper while lowering the melting point. This can save on copper fabrication costs, more so on energy expenses.
Well, you can use various measurement techniques such as spectroscopy, x-ray, ICP analysis, EDX analysis, and conductivity testing to check copper purity.
Copper Grain Size

Grain size and structure affect copper melting temperature. The grains’ structures vary depending on the alloy which in turn affects the boiling and melting point of copper.
For instance, comparing the melting point of brass and copper, there is a variation due to the grain structure. Ideally, adding any element to copper will ultimately affect the grain structure. The net effect will be a change in the melting temperature of the copper alloy.
To expound on this, let’s look at some of these variables that affect at what temperature copper alloys melt.
Hall-Petch Relationship
Also called grain-boundary strengthening, describes how you can alter metal strength by changing the grain size or crystalline structure. That is to say – metals having small crystalline grain structures are stronger and harder. At the same time, small grains imply the metal will have a low melting point.
Comparing mercury and copper vs. lead and copper, the latter has a low melting point. This is attributed to the small grain size in lead.
The melting point of nickel-copper alloy is higher (1, 455°C) than pure copper (1, 085°C). This is mainly due to the large grain size of nickel.
Grain Boundary Energy
A point where two grains meet is called a grain boundary in a copper metal structure. Small grains imply a weak grain boundary within the copper structure. Therefore, the melting temperature of copper alloy will be low.
It implies that a large grain structure will translate to a higher melting temperature of copper alloy. For instance, the melting point of tinned copper is about 1900°F, which shows a change in the grain structure.
Microstructural Heterogeneity
The final copper material may have different structures or cross-section areas. This leads to different variations when melting copper. Ideally, tiny grains of copper melt faster than larger grains, as we had mentioned earlier.
Again, the structural composition also plays a significant role here. For instance:
- Melting point of copper wire may vary between 1,085 °C to 1,215 °C. It depends on the copper wire grade and shape.
- Melting point of copper tubing can start from 1,085°C to a slightly higher temperature depending on the size and cross-section of the tube.
Dislocation Density
Once you understand the dislocation density of copper compound, you can control the melting point. Usually, low dislocation density implies a higher melting point.
Diffusion Rates
Very small copper particles tend to heat faster than large copper particles. This is quite evident when examining the melting point of copper wires.
The manufacturing process may alter the copper granule sizes. Which will essentially make copper with small grains melt faster.
Surface Energy
Whenever the surface energy changes, expect the melting point of copper to change. Remember, copper will melt once the heat energy supplies breaks the surface energy.
Stress Concentration
Subjecting copper to high stress will lower the melting. This is because stress interferes with the normal arrangement of copper atoms in its structure. Remember, subjecting copper to stress may result in plastic deformation.
In addition to these, there are certain post-fabrication processes such as hardening and recrystallization. Such processes may also alter the copper melting point. They may align, compact, or harden copper to achieve better temperature endurance characteristics.
Surface Area Vs. Copper Melting Point
There are certain studies and research that highlight the significant effect of surface area on the copper melting point. Remember certain heat treatments of copper or its alloys, may affect the atoms on the surface.
Consequently, the temperature at which copper melts after such treatments may show wide variations.
In short, the surface treatment, atom, or particle structure determines the melting temperature of copper and its alloys.
Ways of Melting Copper
You can use different techniques to melt copper. It does not matter whether you are using copper welding rods, sheets, or bars, you need a reliable technique. A process that will make pure copper or copper alloy transition from solid to liquid phase.
With the advancement in technology, there are many copper melting furnace for sale in the market. Let’s look at some common melting technologies:
- Arc melting copper – It requires high electric current due to the high melting and boiling point of copper. With sufficient current, you can achieve 100% copper conversion.
- Induction heating – With the help of oscillators, electromagnets, and high-frequency alternating current, it can effectively heat and melt copper.
- Electric resistance heating – This is a common heating technique for most copper alloys such as brass. As electric current passes through copper alloy it heats up thereby beginning to melt.
- Laser melting – A high-powered laser melts copper to the desired phase.
- Electron beam melting – The technique uses a high-energy electron beam to melt copper metal. These are sufficient enough to elevate copper temperature beyond the melting point.
- Oxy–fuel Torch – A mixture of propane and oxygen mixes to heat copper beyond its melting temperature.
- Furnace heating copper – There are many types of furnaces you can use to heat copper to the melting point. These may include a cupola furnace, gas, or induction furnace.
- Microwave melting – There are many types of microwave assistant furnaces available that effectively heat copper to the melting point.
- Salt bath melting – With the help of cyanide salts and chloride, the system evenly distributes heat around copper for the melting process. This technique works well with anhydrous copper sulphate processing.
- Infrared heating – While minimizing oxidation, the infrared heaters heat copper to melting point.
- Direct electric heating – Current passes through copper thereby generating heat to melt copper. So far, this is a practical technique for most copper alloys. The method is fast and efficient.
- Vacuum melting – With the help of a vacuum chamber, you will melt copper without any possibility of contamination. You can reach high temperatures for melting copper easily.
- Plasma arc melting – With plasma arc you can melt copper rapidly and effectively. It works well with most copper alloys.
- Suction casting – The process uses high heat thereby melting copper. It is a common process when producing intricate copper parts.
- Hot isostatic pressing – It combines both high pressure and temperature to melt copper material.
- Cold crucible melting – With the help of a water-cooled crucible, the process uses electromagnetic induction to melt copper metal.
- Solar furnace melting – The process depends on solar energy to heat solar. It is one of the green energy technologies in the copper melting process.
As you can see, there are many technologies you can use when melting copper metal. It depends on the purity, efficiency, reliability, and energy source availability.
Comparing Melting Points of Other Metals and Copper
Due to the different atomic structures, the melting points of metals vary broadly. In this section, we will look at the melting point of common metals in the industry while comparing them to copper:
| Metal | Melting Point of Metals and Metalloid in °C |
| Copper | 1084 |
| Melting point | 660 |
| Cast iron | 1127 to 1204 |
| Carbon steel | 1371 to 1593 |
| Chromium | 1860 |
| Cobalt | 1495 |
| Incoloy | 1390 to 1425 |
| Inconel | 1390 to 1425 |
| Stainless steel | 1510 |
| Molybdenum | 2620 |
| Magnesium | 349 to 649 |
| Niobium | 2470 |
| Nickel | 1453 |
| Tantalum | 2980 |
| Titanium | 1670 |
| Tungsten | 3400 |
| Thorium | 1750 |
| Rhenium | 3186 |
| Rhodium | 1965 |
| Ruthenium | 2482 |
| Osmium | 3025 |
| Platinum | 1770 |
| Palladium | 1555 |
| Vanadium | 1900 |
| Zirconium | 1854 |
As you can see, copper melts at relatively high temperatures. However, tungsten remains a metal with the highest melting point.
Melting Point of Copper ipe
- 1,981°F/1082°C.
Melting Point of Copper and Aluminium
- Copper – 1,675-1,981°F / 913-1,082°C.
- Aluminum – 1,218 °F / 659 °C
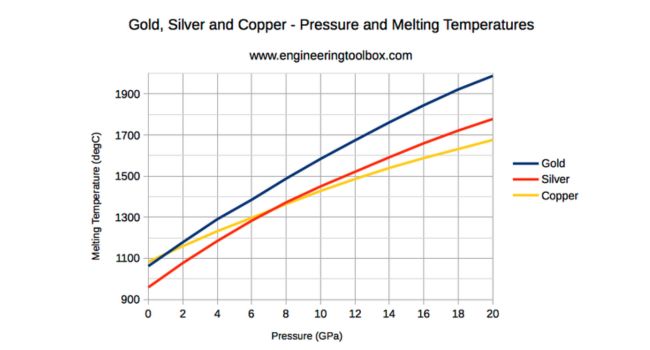
Melting Point of Copper Silver and Gold
- Copper – 1084°C (1983°F)
- Silver – 961°C (1762°F)
- Gold – 1063°C (1945°F)
How to Melt Copper
Since we have covered the critical aspects above, this section will be a quick summary:
- Step 1: Choose tools and equipment – adopt a cost effective copper melting technique
- Step 2: Prepare the copper material you want to melt. You can clean and cut into small pieces.
- Step 3: Ensure you wear safety gear and ventilation should be sufficient.
- Step 4: Put copper material in the melting equipment (furnace). Heat to attain the required copper melting temperature.
- Step 5: Pour molten copper into shapes you wish to make or ingots.
How Copper Melting Point Determine Practical Application
As copper melts, there are drastic changes in the mechanical, physical, and maybe chemical properties. At this point, copper and copper alloys can only lead to component failure.
However, knowing the temperature copper melts will determine the practical applications. Let’s look at a few:
Welding, Soldering, and Brazing
Many copper brazing rods are used in several applications today. Of course, this is possible by knowing the thermal properties of copper. During welding, copper melts thereby helping in the joining process.
Electrical Applications
Copper is known as one of the best conductors. There are many electrical wires, transformer parts, heat exchanger systems, heating systems, generator systems, heat sinks, etc. made from copper materials.
All these designs are based on a comprehensive understanding of the temperature and thermal properties of copper material.
Casting and Molding Applications
Through casting copper, you can make many sculptures, plumbing fittings, jewelry, and automotive parts, among other parts.
During casting operations, the knowledge of the melting temperature of copper will help you choose the right furnace and tooling systems. It is even critical when choosing material for the tooling equipment since it must withstand hot copper material.
Furthermore, it will help you determine the energy required to fabricate copper. This makes a vital component when giving quotations. It also gives the fabricator a chance to analyze certain variables such as the density of copper at melting point.
Conclusion
On average, the melting point of copper is 1,085°C. However, this temperature may vary depending on the copper alloy type and other variables such as crystal structure or impurities.
Additionally, the knowledge will help you determine whether copper is the best material for your application or not.
At KDMFAB, we are your trusted copper parts fabricator in China. For all your fabricated copper parts, contact us now for competitive prices.
More Resources:
Melting point of Titanium – Source: KDM
Aluminum Melting Point – Source: KDM
Steel Melting Point – Source: KDM
Does Copper Rust – Source: KDM




