One critical fact about titanium is – its high melting point or temperature. Well, this is also alongside other fascinating features such as low density and high strength-to-weight ratio.
In this article, we shall explore all important facts about titanium melting point – let’s dive right in:
What Temperature Does Titanium Melt?
Theoretically, the melting point of pure titanium is higher than most metals. That is to say:
- Melting point of titanium in Celsius is 1725°C
- Melting point of titanium in Fahrenheit is 3135°F
As you can see, the melting point of titanium is relatively higher than most common metals as evident in the table below:
| Metal | Metal Melting Point (°C) |
| Aluminum | 660 |
| Brass | 930 to 1000 |
| Cobalt | 1495 |
| Copper | 1084 |
| Lead | 327.5 |
Although titanium’s melting point is high, there are metals with higher melting temperatures. A good example is tungsten. The melting point of tungsten is 3422°C. It is nearly twice the melting temperature of titanium.
Additionally, other melting points of metals that have higher titanium melting temperatures are:
- Rhodium – 1963 °C
- Tantalum – 3020 °C
- Iridium – 2446 °C
- Thorium – 1755 °C
- Vanadium – 1910 °C
Basic Information about Titanium
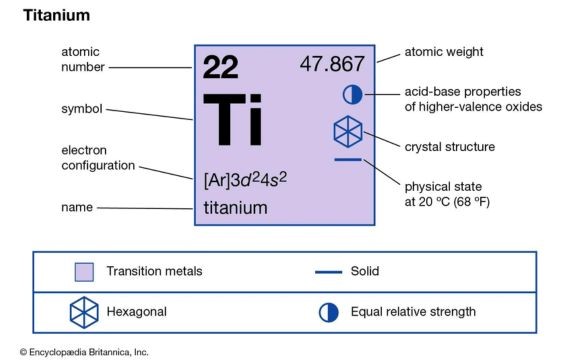
Titanium is among the transitional metals known for its high melting point. In the periodic table, titanium (Ti) is atomic number 22. Furthermore, titanium mainly exists mostly as ilmenite and rutile.
A combination of low density, high strength, and high melting point makes it a perfect material for many applications. It was among the popular metals that were used during the Soviet Union in the aerospace industry between the 1950s and 1960s.
Factors Affecting Melting Temperature of Titanium
Again, as you explore the melting temperature of titanium, you will realize that in its pure form, this metal begins to melt at 1725°C. However, you may realize some variations depending on the level of purity.
For example:
If you change the diffusion mobility of atoms in titanium, the melting point may change by 450 °C. It is actually for this reason that you will find some titanium alloys having higher melting points.
Let’s quickly look at examples of the most common melting point of titanium alloy:
| Example of Titanium Alloy | Titanium Alloy Melting Point (°C) |
| Ti 6AL-4V | 1878 – 1933 |
| Ti 6AL ELI | 1604 – 1660 |
| Ti 3Al 2.5 | ≤ 1700 |
| Ti 5Al-2.5S | ≤ 1590 |
Remember, there are processes such as dispersion strengthening that can greatly improve titanium melting point.
Reason Why Titanium Melting Point is High
Generally, the melting point of metal or any material depends mainly on the atomic structure or chemical bonds.
Ideally, titanium atoms have a strong chemical bond, which makes them resistant to high temperatures.
Unlike other metals, the atomic weight of titanium is high. Besides, it has a valence of 4. Remember, when an element has a higher atomic weight, its atoms will not vibrate easily.
Since titanium has a higher atomic weight, it implies the atom will not loosen easily from the matrix. Therefore, you will require more thermal energy to loosen the atom and hence transition from solid to liquid (melting titanium).
Another fact is the titanium valence. Remember, it is the valence that determines the binding of electrons in an element. An element with higher valence implies an electron can bind easily on the atom.
And, if there is more binding, it implies you will require more thermal energy to loosen. This implies the material begins to melt.
Another critical thermal property of titanium is low thermal expansion. That is to say, whenever you heat titanium, the expansion and contraction are quite small.
Titanium’s low thermal expansion makes the material dimensionally stable. It is for this reason that titanium parts are common in many industries due to dimensional stability.
Thermal Properties of Titanium vs. Applications
Titanium plays an integral role in many applications due to its high melting point and other characteristics. Let’s look at some common applications:
As a Material in Aircraft and Missile Construction
Titanium alloys combine some desirable properties for aircraft, missile, and rocket parts construction. This is mainly attributed to superior thermal properties including the high melting point. Besides, the material is tough and lightweight.
Functions as a Refractory Metal
Titanium melting point is about 1,668 °C, which makes it a perfect refractory metal. Remember, refractory metals are known for their superior resistance to extreme heat and wear.
Titanium Heat Exchanger
Titanium heat heat-resistant property due to high temperature makes it a perfect material for heat exchanger systems. There are many industrial tubing made from titanium alloys due to the high melting point.
Both titanium shell and titanium tube can handle highly corrosive fluids even and high pressure and temperature.
Turbine Engine
A turbine engine should have high strength and high-temperature resistance. This makes titanium turbine engines a perfect choice for most applications.
Melting Point of Titanium vs Stainless Steel
Titanium melting point of 1650–1670 °C (3000–3040 °F).
Stainless steel melting point of 1230–1530 °C (2250–2790°F).
Conclusion
As you can see, titanium’s melting temperature is higher than most common metals. It is for this reason that titanium is popular in aerospace, heating, and military applications.
For all your titanium fabricated parts, KDMFAB is your trusted partner – contact us now.
More Resources:
Does Titanium Rust? Source: KDMfab
Titanium Metal – Source: IQS Directory




