In this article, you are going to learn everything about the melting temperature of iron, why it is important and even compare it to the melting point of other metals.
Later, you will learn how to melt iron and factors affecting the melting point.
Let’s dive right in:
What is Iron Melting Point?
Iron melting point is referred to as the temperature at which iron changes change from a solid state to a liquid state.
| Unit of Measurement | Values |
| Melting point of iron in Celsius | 1538°C |
| Melting point of iron in Fahrenheit | 2800°F |
| Melting point of iron in Kelvin | 1811 Kelvin |
Reasons for Knowing Melting Temperature of Iron
Here are some important reasons why iron’s melting temperature should be known:
- In ensuring processes like welding, forging, and casting are well optimized.
- To ensure iron is chosen for ideal and right uses that involve higher temperatures.
- Knowing iron’s melting temperature prevents structure failures in setups where higher temperatures are anticipated.
- To design and manufacture alloys that are iron-based and have unique properties.
- For planning heat treatment methods for specific mechanical properties to be achieved.
How Iron Melting Point compare to other Metal Melting Point
Iron melting point at 1538°C is not the same as other metals. This is why knowing these differences help. Below are the different points for other temperatures:
| Metals | Metal Melting Point in degrees Celsius |
| Melting point of steel | Ranges from 1370°C to 1510°C based on its composition |
| Melting point of copper | 1085°C |
| Melting point of gold | 1064°C |
| Melting point of lead | 327°C |
| Melting point of brass | Ranges from 900°C to 940°C based on its composition |
| Melting point of titanium | 1668°C |
| Melting point of silver | 961°C |
Melting Point of Different Types of Iron
Although iron’s melting point temperature is at 1538°C, different types of iron will come with different melting points. These are made available below:
| Type of Iron | Iron Melting Point in degrees Celsius |
| Melting point of Cast iron | 1150 to 1200°C |
| Melting point of wrought iron | 1482 to 1593°C |
| Melting point of white iron | 1130 to 1350°C |
| Melting point grey iron | 1150 to 1200°C |
| Melting point of ductile iron | 1150 to 1200°C |
| Melting point of malleable iron | 1170 to 1350°C |
Boiling Point of Iron vs. Melting Temperature of Iron
The boiling point of iron is 2862°C while its melting point is 1538°C.
Here, the melting temperature has to do with the temperature where solid iron is transformed into liquid. On the other hand, the boiling point of iron has to do with the temperature where liquid iron is turned into gas.
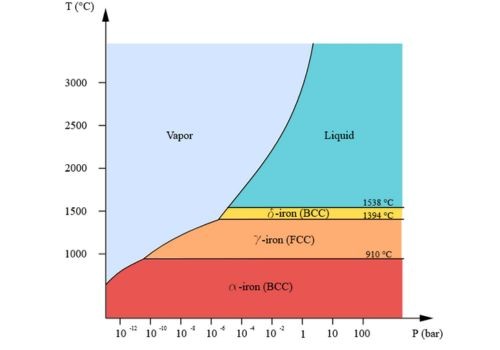
Factors Affecting Melting Point of Iron
Below are some factors that affect iron melting point. These include:
- Melting point of iron can go higher or come lower when there is the presence of impurities such as carbon.
- Heightened pressure can increase melting point even as decreased pressure reduces it.
- When alloy elements like nickel, chromium, or manganese are added, it alters iron melting temperature.
- Annealing methods can affect iron internal structure and this can affect iron melting point.
- The size of grains can affect slightly iron melting point. This is due to their power on the stability of total material.
- Unique allotropes of iron such as austenite and ferrite come with different melting points.
How Melting Iron Affect its’ Properties
There are different ways by which properties of iron are affected. These impacts touch the chemical, physical, and mechanical properties. Below are these effects:
- Iron phase is changed from solid to liquid.
- Iron volume expands when it melts. This reduces its density.
- Iron’s thermal conductivity is reduced when it is in its liquid state than in its solid state. This affects its heat distribution.
- Iron had low electrical conductivity properties when it is in liquid state, the electrical conductivity properties of iron is lower.
- Liquid iron viscosity is less compared to solid iron. This makes molding or casting super easy.
- The droplet forming and spreading of liquid iron with regards to surface tension is affected. This is valuable in methods such as casting or welding.
- The mechanical strength of liquid iron and its rigidity goes away. However, these properties are regained when iron solidifies.
- With other elements, molten iron is highly reactive. This leads to oxidation and higher potential for alloys to form.
How to Melt Iron

Iron melting involves unique steps that are undergone in specific environments. The process is below:
Step 1 – Iron ore or Scrap Obtained
Raw materials like scrap or iron ore is obtained.
Step 2 – Get Rid of Contaminants
Make sure your raw materials have no impurities or contaminants.
Step 3 – Iron Furnace Type
Here, the specific furnace you will prefer to use should be chosen. You can choose from cupola, blast, electric arc, or induction furnaces. All these will meet specific needs.
Step 4 – Furnace Charging
Put in raw materials by loading them into the furnace. This should be done with limestone, coke or any fluxing agent also added to take out any contaminants.
Step 5 – Heat Raw Materials
Begin heating by turning the furnace on and steadily increasing temperature around 1538°C – its ideal melting point. Ensure heating is consistent to make melting even.
Step 6 – Monitoring
Make sure you keep an eye on the melting process to make sure iron gets to a molten state that is complete. Ensure it doesn’t overheat. This should be done by adjusting flux and temperature when needed.
Step 7 – Take Contaminants Out
Ad melting is going on in step 6, contaminants form a slag on its surface. These impurities must be removed to keep molten iron purity intact.
Step 8 – Tap Molten Iron
When iron ore or scrap is fully molten, tap furnace to transfer molten tin into ladles or molds. When pouring, make sure its flow is controlled to prevent splashes.
Step 9 – Cooling
Leave molten iron in molds or ladles to cool and become solid in ideal form or shape. If there is the need for controlled cooling or annealing, it should be done here.
Step 10 – Check its Quality
Ensure it is checked for quality and consistency. Also, you can have it tested.
Why is Melting Point of Iron so high?
Due to the strong metallic bonds present between iron atoms, high amounts of heat is needed to break the bonds to liquid from solid. This leads to its high melting point.
Conclusion
As you can see, the melting point of iron is slightly higher compared to other metals. This explains why iron alloys have relatively higher melting point.
At KDM, we are a leading metal fabrication company in China. Depending on your specific needs, we will choose a perfect metal alloy for your fabrication needs.




