Metals need corrosion resistance for many applications to maintain their performance over time, staying unaffected by environmental exposure.
Degradation or corrosion won’t harm them. “Corrosion resistance” is a crucial term. Metals can resist corrosion and restore damaged surfaces.
Different materials show this quality differently. Alloy composition is one way. Galvanizing is another. Anodization also works. “Metals that are corrosion-resistant” is the main motto of this post. We will explore more about them.
The Science Behind Corrosion Resistance!
Understanding the science behind corrosion resistance is crucial, and it helps us grasp the mechanisms involved in preventing metal degradation.
Corrosion involves electron transfer between the metal and the environment. Here are some key points to consider:
1. Electrochemical Process of Corrosion
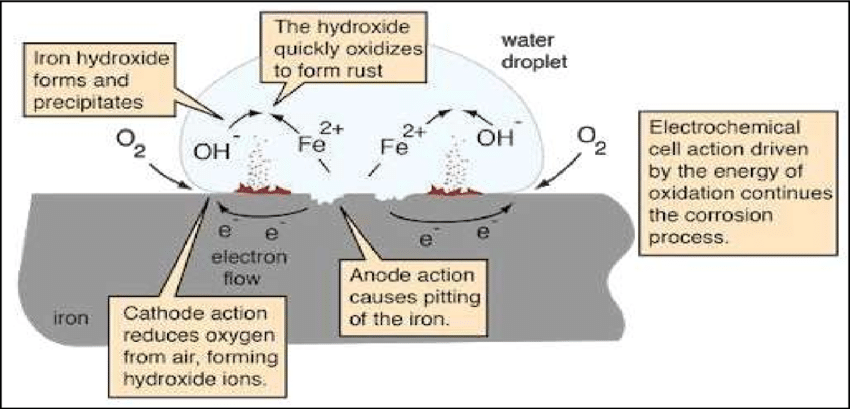
Figure 1 – Electrochemical Process of Corrosion
Corrosion is an electrochemical process that involves electron flow from the anode to the cathode. Metals exposed to a reactive environment corrode.
Examples include saltwater or acid rain. However, the anode corrodes, releasing metal ions, and these ions combine with the environment, forming a new substance. By continuing this process, further damage is caused to the metal and its surroundings.
Corrosion can be accelerated by factors, such as high temperatures, humidity, and acidity. When exposed to saltwater, metals corrode faster.
Salt ions increase the surrounding medium’s conductivity. Impurities in the metal or environment can cause galvanic corrosion.
| Process Stage | Description | Factors Affecting Corrosion | Examples |
| Anode Corrosion | Electron flow from anode to cathode; anode corrodes and releases ions | High temperature | Saltwater, acid rain |
| Formation of Substance | Metal ions combine with the environment, forming a new substance | Humidity | Galvanic corrosion |
| Further Damage | New substance causes further damage to the metal and surroundings | Acidity | Dissimilar metals in contact |
| Acceleration | Various factors can affect | Impurities, salt ions | Saltwater exposure |
Overview of the Electrochemical Process of Corrosion
2. Factors Affecting Corrosion Resistance

Figure 2 – Rusty Metal Affected By Environmental Exposure
Below are factors that can lead to corrosion:
§ Material Composition
Metal’s composition affects its corrosion resistance. Alloying elements play a role. Adding chromium to steel increases its resistance.
§ Environmental Exposure
Environment affects corrosion resistance. Metals in saltwater or acidic environments corrode more quickly. Less corrosive environments have less impact.
§ Surface Finish
Metal’s surface finish is important. Rough surfaces trap contaminants, increasing corrosion likelihood.
§ Corrosion Resistance Ratings
Needless to say, the industry has corrosion resistance ratings for metals based on their performance in specific environments.
§ Temperature Limitations
Some metals have temperature limitations that affect their corrosion resistance.
§ Cost
Metal’s cost can be a factor, but the application of the metal and whether it rusts also play an important role in influencing which one is chosen; often the cheapest metal that doesn’t corrode will be preferred.
§ Maintenance and Replacement Considerations
Ease of maintenance and replacement matter when selecting a corrosion-resistant metal.
Top Corrosion-Resistant Metals!

Figure 3 – Metals that Are not Affected by Corrosion
Corrosion is a major issue for metal objects exposed to open air. Degradation of investments in metals can occur. Various effects and risks are associated with this, such as decreased strength and risk of injury from falling pieces. Consider these metals for your projects and designs.
Here is a list of top metals with high rust resistance:
§ Stainless Steel
Stainless steel is among the least reactive, most utilitarian metals. Due to its low carbon content, it has maximum corrosion resistance and is cost-effective compared to other alternatives.
Stainless steel is perfect for a rust-free option, making it ideal for food processing and medical applications.
With the right environment and alloy grades, it can last over 100 years without rusting.
§ Aluminum and Aluminum Alloys
Aluminum is typically lightweight with good strength-to-weight ratios, what makes it an attractive building material. A well-made aluminum alloy is highly corrosion-resistant.
Although Aluminum is among the cheapest metals that don’t rust, it is not completely “rust-proof” and air becomes more corrosive over time due to oxidation.
§ Titanium and Titanium Alloys
Titanium alloys withstand the wear and tear of harsh conditions. You can trust titanium alloys to have good corrosion resistance, because they have good strength-to-weight ratios.
Ideal for marine, aerospace and medical applications where rust is a concern, these materials offer superior corrosion resistance.
§ Copper and Copper Alloys
Metals like copper resist corrosion even after prolonged contact with water or moisture.
Copper is used in shipbuilding as part of a protection system to increase the longevity of vessels. Vessels can survive more time without rusting if correctly maintained.
§ Nickel and Nickel Alloys
Nickel resists high temperatures and strong air pressure, which makes it ideal for the auto industry in exhaust systems.
Its corrosion-resistant characteristics remain when compounded with chromium to help prevent rust. Nickel is a great option for offshore applications.
All these metals have strengths and weaknesses. Knowing what you are dealing with is important. Make your decision on the suitable metal for your project accordingly.
| Metal | Key Characteristics | Common Applications | Rust Resistance | Cost-effectiveness |
| Stainless Steel | Low carbon content, least reactive | Food processing, medical | High, up to 100 years | High |
| Aluminum & Alloys | Lightweight, good strength-to-weight ratio | Building materials, outdoor applications | Moderate to high | High |
| Titanium & Alloys | Excellent chemical resistance, good strength-to-weight ratio | Marine, aerospace, medical | High | Moderate |
| Copper & Alloys | Long-term resistance, resists water/moisture | Shipbuilding, vessel protection | High | Moderate |
| Nickel & Alloys | High temperature & air pressure resistant | Auto industry, offshore applications | High | Moderate |
Corrosion Proof Metals
Coatings and Treatments to Enhance Corrosion Resistance!

Figure 4 – Metal Coatings and Treatments
Coatings and treatments are applied to metals often to improve corrosion resistance. But a barrier is created and lies between the metal surface and the environment. Here are common coatings and treatments:
§ Galvanizing
Galvanizing is a process that coats iron or steel with zinc. Therefore, instead of the metal corroding, it is the zinc coating that undergoes corrosion. Galvanizing is used in harsh environments.
Examples are marine or industrial settings. However, the coating is cost-effective, and popular for cost-sensitive applications.
§ Anodizing
Anodizing is an electrochemical process that increases corrosion resistance.
By immersing aluminum in an electrolyte solution and then passing an electrical current through the metal, a process happens that forms an oxide layer.
Anodizing can add color and improve the appearance, which is important for metal in architectural applications.
§ Electroplating
Electroplating involves coating a metal. A thin layer of another metal is used. An electrochemical process is used.
Electroplating can coat various metals, which can improve corrosion resistance, wear resistance, hardness and appearance.
Copper, nickel, chromium and gold are examples of the coating which is used in automotive and aerospace industries.
§ Powder Coating
Powder coating is a process which applies dry powder to a metal surface, and the metal is then heated in order to create a durable coating.
Particles of polymer resin are given an electric charge, then sprayed onto the metal before it is heated in a furnace.
Powder coating is used for durability and resistance, however it melts and forms a hard coating which is important for outdoor furniture and automotive parts.
§ Organic Coatings
Organic coatings are made from organic materials. Examples are resins, oils, and waxes.
Coatings improve corrosion resistance, and can be applied to various metals. Organic coatings are applied by spraying, brushing, or dipping.
Metal coatings available in different colors and finishes are used in architectural applications, with the metal appearance being of particular importance.
| Coating/Treatment | Method | Key Benefits | Typical Applications | Examples of Materials |
| Galvanizing | Coating iron or steel with zinc | Cost-effective, sacrificial anode protection | Marine, industrial settings | Zinc |
| Anodizing | Electrochemical process on aluminum | Increased corrosion resistance, color, appearance | Architectural applications | Aluminum oxide |
| Electroplating | Electrochemical process with various metals | Corrosion, wear resistance, hardness, appearance | Automotive, aerospace industries | Copper, nickel, chromium, gold |
| Powder Coating | Applying dry powder to metal surface | Durability, resistance | Outdoor furniture, automotive parts | Polymer resin |
| Organic Coatings | Spraying, brushing, or dipping | Corrosion resistance, color, finishes | Architectural applications | Resins, oils, waxes |
Common Coatings and Treatments for Enhancing Corrosion Resistance
There isn’t a single cheapest metal that doesn’t rust. However, some metals are more resistant to rust. Stainless steel is an example that does not rust easily.
Aluminum is rust-proof too. Metals like these do not rust in water, and even are corrosion-resistant materials.
How To Choose Corrosion-Resistant Metals?

Figure 5 – Selection Process for Corrosion-Resistant Metals
When choosing corrosion-resistant metals, several factors must be considered to ensure the metal’s optimal performance in a given environment. These factors include:
§ Material Composition
Most alloys contain materials that make them withstand any form of corrosion.
Pure metals have lower resistance. Consider the intended application and corrosive environment to determine the appropriate alloy.
§ Environmental Exposure
Corrosive environment is critical when selecting corrosion-resistant metals. Factors such as temperature, humidity, pH, and pollutants can affect the rate of corrosion.
Metals exposed to saltwater or acidic environments may require higher corrosion resistance. Those used in less corrosive environments may not.
§ Surface Finish
Surface finish affects corrosion resistance. Smooth and polished surfaces are less prone to corrosion. Rough or uneven surfaces are more prone.
§ Corrosion Resistance Ratings
Different metals have varying corrosion resistance degrees. Standardized tests like ASTM G48 measure this.
However, these tests provide information on susceptibility to different corrosion types. Consider the metal’s corrosion resistance ratings when selecting a metal for the intended application.
§ Temperature Limitations
Some metals exhibit corrosion resistance at ambient temperatures, and may corrode rapidly at higher temperatures.
Consider the temperature range when selecting a metal. Ensure it maintains its corrosion resistance properties.
§ Cost
Cost of the metal is crucial when selecting corrosion-resistant metals. Some metals, like titanium and nickel alloys, offer superior corrosion resistance.
However, they may be expensive, and may not be suitable for cost-effective applications.
§ Availability and Lead Time
Availability and lead time are critical factors when selecting corrosion-resistant metals. Some metals may be readily available. Others may require a longer lead time, affecting project timelines.
§ Maintenance and Replacement Considerations
Consider maintenance and replacement requirements when selecting corrosion-resistant metals. Some metals may require regular maintenance, including cleaning or coating to maintain corrosion resistance properties.
Metals that are difficult to replace or repair may not be suitable for applications with critical downtime.
Consider the intended application, environmental exposure, surface finish, corrosion resistance ratings, temperature limitations, cost, availability and lead time, and maintenance and replacement considerations when choosing corrosion-resistant metals.
Careful consideration of these factors leads to longer service life and reduced maintenance costs, helping ensure the selected metal performs optimally in the given environment.
Challenges and Future Trends in Corrosion-Resistant Metals!
In the present era, rapid industrialization and technological advances occur. Corrosion-resistant metals are essential for multiple industries.
All these materials possess strong physical properties, and are suitable for use in harsh environments such as marine applications.
Also, they are useful in environments with highly corrosive substances, but these materials have limitations. Proper maintenance is required to ensure optimal performance.
A number of challenges must be addressed in order to realize the full potential of corrosion-resistant metals worldwide.
§ Nanotechnology and Corrosion Resistance
Nanomaterials are more resistant to corrosion and offer greater mechanical strength and a higher young’s modulus than conventional materials.
By improving chemical resistance properties and having superior electrical conductivity, they have slowed down the corrosion process.
Nanomaterials are used to develop coatings and surface treatments which protect against corrosive agents such as salt water or acid rain.
Researchers worldwide are developing and testing new materials which are more durable than traditional coatings or conventional protective measures such as painting.
§ New Alloys and Materials with Improved Corrosion Resistance
Specialized alloys have improved corrosion resistance. 7075-T6 aluminum alloy is considered to be very durable and weatherproof.
Stainless steel has unique features, with excellent fracture toughness and corrosion resistance.
Such features include cold formability and superior thermal conductivity that make it ideal for applications in different industries.
§ Recycling and Reuse of Corrosion-Resistant Metals
Recycling preserves the environment by reducing costs associated with disposal or production and supports sustainable development by utilizing industrial waste efficiently.
Recycling and reuse of corrosion-resistant metals prevent waste, providing a source for further industrialization.
According to the US Environmental Protection Agency (EPA), in 2019, plastic composites with at least 75% recycled content made up just over 8 million tons of recycled products.
Most importantly, this trend is seen with other corrosion-resistant metals too. Substantial effort and research are invested to make them recyclable.
§ Reducing Environmental Impact during Production and Use
Producing corrosion-resistant metals has a significant environmental impact. Toxic chemicals, water consumption, energy demands, and waste management are involved.
Also, the industry needs more sustainable practices and must reduce greenhouse gas emissions significantly.
Best management practices should be adopted for sustainable resource use and efficient waste disposal. Improved product standards are needed to ensure minimal impact on climate change.
§ Non-Destructive Testing Methods for Corrosion Detection
Accurate corrosion detection is essential, and helps manage the maintenance and repair process properly.
Non-destructive testing methods provide reliable information about material performance, but they do not damage the sample being tested.
Such methods enable timely action for necessary repairs or replacements, increasing safety and improving asset performance.
§ Remote Monitoring and Predictive Maintenance
Thanks to the power of the Internet of Things (IoT), predictive maintenance has undergone a transformative change. Real-time monitoring of corrosion-resistant metals has become possible, allowing engineers to spot early signs of wear or damage before they become critical.
By quickly recognizing areas in need of attention, professionals can take prompt action while conserving time and resources.
Conclusion
At the end of the day, choosing a material or alloy for your project that is corrosion-resistant and doesn’t rust may seem daunting. However, with this comprehensive guide in hand offering an overview of these materials along with their properties such as malleability and cost, it can be simple for you to make the right decision.
Along with this guide, KDMFab would be more than happy to help come up with a solution tailored specifically toward your application’s needs at www.kdmfab.com. We are here to answer any questions you may have and provide a quotation for your project needs in no time!
At KDMFAB, we pride ourselves on our attention to detail, exceptional customer service, and extensive knowledge in the field of corrosion-resistant materials.