If you ask an average person “what is anodizing”, they likely wouldn‘t know what it means. Anodizing is an electrochemical process that alters metal surfaces to create a thin layer.
Not to mention, this layer has increased durability, mainly used on aluminum and stainless steel. Known as aluminum oxide coating, it has been around since the mid-20th century.
Explaining the technique, this article covers its definition, types, applications and process stages.
How Anodizing Works?
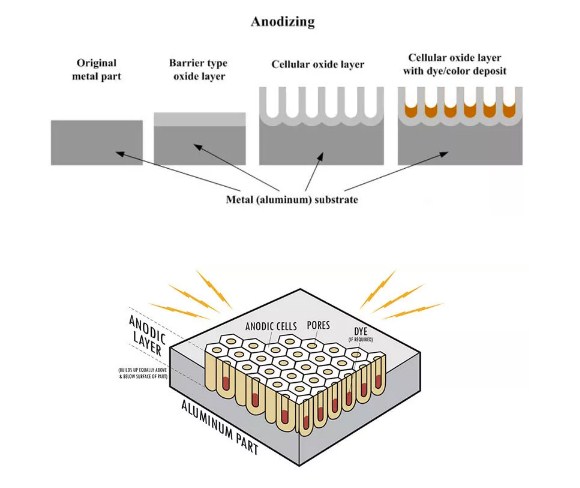
Figure 1 – Anodizing Working System
Many people don’t understand how anodizing works and how to tell if aluminum is anodized or its uses. Here is a guide on how it works and its uses.
Anodizing creates an oxide layer on metals. Material is placed in a chemical solution and electricity passes through it, creating a protective coating.
Process of hardening improves both durability and appearance, creating a surface that is resistant to wear, corrosion, chemicals and heat.
Anodizing has two main steps. First, the metal substrate is chemically cleaned. Then, an electrochemical reaction occurs. An electric current creates an oxide film on the surface and oxygen molecules react with metallic ions to form new atomic bonds between them.
A hard protective layer surrounds the metal substrate. Metal ions from the alloy can be incorporated into the coating, and its oxide layer prevents chemical corrosion whilst being electrically insulating.
An anodized surface can be decorative and has superior protection from oxidation, coupled with a tough outer coating that doesn‘t scratch off easily; its new surface also has a strong molecular bond with the substrate.
Types Of Anodizing
Various anodizing types exist in the metal finishing industry. Each offers unique benefits for specific projects and applications.
Sulfuric acid anodizing, chromic acid anodizing, and hard anodizing are available. We will discuss the differences and best uses for each type.
· Sulfuric Acid Anodizing
Sulfuric acid anodizing is economical and widely used for aluminum finishing. Known as conventional or Type II anodize, it has many applications in industries such as consumer, aerospace and industrial.
KDMFAB commonly uses this method. Thickness ranges from 0 to 15 µm (0–1/16 inch) and sulfuric acid anodizing uses sulfuric acid as the electrolyte. The acid bath is cost-effective and easy to control but not temperature-dependent.
Turn off the power and the bath goes dormant, eliminating the need for equipment like electroplating or etching baths. Anodizing improves the corrosion resistance and seals aluminum surfaces with a hard oxide layer on alloys.
Dyes can be used for both decorative coloring and identification of part surfaces based on end applications. Thick transparent layers generate static control of sliding surfaces and are hard and slippery.
· Chromic Acid Anodizing
Type I or chromate conversion coating uses baths of acids like sulfate or chromic to create Chromic acid anodizing.
Such finish offers solid metallization, better coloration, and coating uniformity. Chromic acid anodizing provides ultra-thin layers on zinc or aluminum.
Thickness ranges from 0 to 25 nm (1/1000 inch) is ideal for products needing strength, like in aircraft operating conditions.
Chromic acid anodizing enhances corrosion protection for aluminum and zinc surfaces for providing greater electrical insulation properties.
· Hard Anodizing
Conventional methods produce thinner layers than “sulfuric acid anodize“ Type III or “sealed”, which is also called Hard Anodizing.
For projects requiring high strength and hard surfaces, this method suits the best as it requires thick electrodes for high voltages and lubricative capabilities. Electrolyte has easy maintenance, good electrical and thermal conductivity.
Hard anodizing involves metallurgical bonding and the use of sulfuric acid–electrolyte with a higher voltage current, generally ranging from 18 to 30V.
Hard anodizing offers a wide range of colors and finishes varying from metallic to almost opaque black, making it suitable for components in aerospace, automotive and naval engineering applications.
| Anodizing Type | Thickness Range | Key Applications | Benefits | Common Industries |
| Sulfuric Acid | 0 to 15 µm (0-1/16 inch) | Aluminum finishing | Cost-effective, improved corrosion resistance, versatile dyeing options, hard and slippery layers | Consumer, Aerospace, Industrial |
| Chromic Acid | 0 to 25 nm (1/1000 inch) | Products needing strength | Better coloration, solid metallization, uniform coatings, enhanced corrosion protection, better electrical insulation properties, better adhesion | High volume production, Aerospace |
| Hard Anodizing | Thicker than conventional | Components needing high strength, hard surfaces | Enhanced wear and abrasion resistance, wide range of colors, good electrical and thermal conductivity | Aerospace, Automotive, Naval Engineering |
Comparison of Anodizing Types
Anodization is a crucial metal forming technique and used for industrial and decorative purposes. Understanding different anodizing types helps determine the best option for your project.
Advantages Of Anodizing!
Figure 3 – Anodizing Benefits
Anodizing is low-cost compared to other coatings that offer benefits like corrosion resistance and durability. Enhanced adhesion for paints and dyes is also a plus, which allows achieving aesthetic effects on some alloys. Let’s explore these advantages further.
Improved Corrosion Resistance:
Anodizing improves corrosion resistance, and uncoated materials need special anti–corrosion tactics.
A thin oxide layer forms on the surface, preventing moisture from entering the material and enhancing durability. Anodized surfaces are resilient and wear-resistant.
Anodized surfaces corrode slower than aluminum in exterior applications, especially in humid climates or coastal areas, to extend corrosion resistance.
Enhanced Durability:
Anodic films are durable compared to regular coatings and anodizing provides protection against environmental elements that can withstand everyday wear as well as preventing oxidation after treatment.
However, this property is important for products with exposed surfaces, and machined or cast parts require a durable finish. Anodizing fulfills the need of both aesthetic and practical requirements; some anodizing types focus even further on adhesion and corrosion resistance.
Products needing wear protection and longevity benefit from anodizing, while others provide an improved aesthetic finish.
Better Adhesion For Paints & Dyes:
Anodic films improve adhesion of coatings like paints or dyes, providing a foundation for colored finishes to be added over the anodized layers. Techniques like acid dye add different colors, resulting in better–looking products.
Vivid hues are created without obscuring component structure, and anodized surfaces accept paint and organic coatings well.
Hard-anodizing provides better adhesion than pre-painted aluminum or steel. Colored anodizing minimizes surface prep and cleaning lowering the risk of corrosion from neglected spots.
Aesthetic Appeal:
Anodizing aluminum increases the appeal, and can be used in architectural features or small metal components. Anodic coloring doesn’t fade or degrade under certain conditions.
UV light exposure won‘t affect anodic films like regular painted coatings, but they are durable and protect from environmental elements.
Many firms offer anodizing services which are suitable for outdoor furnishings and light fixtures, and intricate designs can be etched onto aluminum and other metals for visual effects.
Variety Of Materials:
Anodic films can be applied to different materials such as anodized stainless steel, aluminum and titanium.
Many projects need hard–anodizing, a process that works on aluminum and other metals; however, painting and other coatings may not provide good wear protection. These films offer alternative solutions for some metals.
Application of anodic films can be done in several ways, such as immersion into electrolytic baths and process coatings, even on stainless steel.
Special techniques like military-grade anodizing improve wear resistance and enhance component durability.
Lower Cost:
Anodizing comes in various forms and is used via fast, batch, or bulk processes depending on performance needs. Anodizing is often more cost–effective than other coatings such as powder or spray painting and electroplating.
Critical components might need higher protection levels, and anodizing could still be the most affordable choice; many firms offer anodic films with reasonable lead times.
Anodizing is a relatively low–cost solution, and some providers offer it on same day basis which makes them great for fast prototyping and manufacturing.
Environmentally Friendly:
Anodizing is an eco-friendly option and has less pollution as well as hazardous byproducts than other methods.
Anodic films do not need special disposal, and they use safe processes and materials like aluminum. In-house treatment reduces production time and energy consumption.
Most anodizing operations use aluminum-based electrolyte baths. You can learn more about the process and risks online.
Anodic films have many advantages and extend corrosion resistance compared to other coatings. Contact anodizing services to learn more by visiting KDMFab for expert advice on anodizing vs electroplating, painted coatings, and anodized steel and other metals.
Applications Of Anodizing
Figure 4 – Anodizing Applications
What is anodizing? No more unknown to you now, it requires an electric current to pass through metal which creates oxidation layers on the surface. Initially, it was used for corrosion-resistant coatings on aluminum and other metals.
Now, it offers more uses due to the strength, color, and variety of coatings. Popularity has increased across many industries.
Protective aluminum oxide layers are hard-wearing and prevent corrosion. Applications of anodizing are seen in aerospace, automotive, sports industries and architecture & construction.
§ Aerospace Industry
Aerospace industry needs reliable components with highly resistant protective coatings to cope with extreme temperature variations.
Aluminum is important for its weight and strength balance, making it suitable for parts under heavy stress such as wing–frames and engines. Due to aluminum’s high heat conductivity, anodized parts can last longer in regular and extreme environments.
Titanium anodizing provides strength, protection, and beautifying options. Coating processes for aluminum parts create distinctive markings for identification.
§ Automotive Industry
Automotive industry relies on anodizing for protective components. Manufacturers use aluminum in the production process as well as in body parts, panels, frames, and structures.
Anodizing provides a scratch–resistant surface finish and the layer hardness depends on the alloy and treatment.
Anodized surfaces increase component durability and consistent colored visuals are achieved through dyes, with hardcoat and Alocrom processes being examples.
Coatings offer decorative and functional surfaces, enabling components to perform better and last longer. Maintenance costs are lower and aesthetics increase, while colored product features can be achieved with anodizing.
§ Architecture And Construction Industry
Architectural projects need special coatings on aluminum surfaces as they provide corrosion protection and aesthetic enhancement.
Anodizing is the preferred option and has greater weather resistance and improved durability. Anodized surfaces resist sun‘s UV rays, road chemicals and dust/soil particles creating a “honeycomb“ effect.
Weather resistance is improved for outdoor use and discoloration, as well as cracking are reduced compared to paint or powder coating. Anodizing coatings offer a variety of colors that makes it popular for modern building designs and exterior facade wall claddings.
§ Consumer Electronics Industry
Anodized surfaces have strong, hard-wearing properties that are often used in consumer electronics. Rigorous testing occurs in product production, like mobile phones.
Anodizing protects softer alloys like aluminum and creates a hard, durable layer with various color options for pleasing finishes.
Low–frequency electromagnetic waves and electrostatic charge can cause damage to unprotected devices in harsh environments.
Anodized parts serve as a protective barrier and reduce damage. An aluminum alloy casing is combined with die-cast and hot formed processes.
Aerospace components also use anodized parts, because they help identify products through distinct surface markings.
§ Sports And Recreation Industry
Anodizing is useful in the sports and recreation industry due to its anti–oxidizing properties. Engraved components for sports equipment have advantages, and anodizing offers design potential with intricate details.
Modern sport production and customization trends require this. Process–treated parts need resistance against wear and tear, such as engine frames and external components, while ski machinery experiences stress from water materials.
Anodizing offers strong, attractive benefits across industries and its effects are measured scientifically through tests that evaluate abrasion resistance, boiling point, and scalding points.
Anodizing and its potential benefits should always be considered, taking into account factors such as corrosion resistance to help judge the effects before production.
For car protection, aeronautic part manufacturing, and sports equipment design, you can seek advice from KDMFab to learn about anodizing aluminum at home!

Anodizing Vs. Other Metal Finishing Processes!
You may have heard of anodizing. What is it exactly? How does it compare to other metal finishing processes? We‘re here to answer these questions and ensure you get the best results for your product.
Let’s examine the differences between anodizing, electroplating, powder coating, and painting.
· Comparison With Electroplating:
Electroplating applies a thin, protective layer. Layer is electrically conductive material and applied to metal surfaces like copper and zinc.
Additionally, the coating serves an aesthetic purpose that provides corrosion resistance but it doesn’t withstand long-term exposure because sun, rain and snow can affect it.
Electroplating takes upto 16 days for completion, making it more time–consuming than other processes and thus the main comparison point.
On the flip side, it has two major advantages – working on a wide range of materials at less cost – and its main disadvantage is its thickness.
· Comparison With Powder Coating:
Powder coating is a dry-finishing process for creating strong, lightweight layers that protect metal surfaces like aluminum and steel.
Process is cost-effective and suitable for various metal finishing applications, and offers product protection and decorative enhancement.
One advantage over anodizing is coloristic benefits. Yet, it can’t match anodizing’s salt-spray corrosion protection.
A major drawback is color duplication. Colors can be difficult to reproduce, especially for lighter shades, and color consistency can be a potential issue.
· Comparison With Painting:
Painting is accessible and versatile, and its main purpose being long–term protection, it‘s used in both industrial production and art installations. Painting‘s biggest limitation is low durability and has low UV light resistance, and it is also sensitive to environmental conditions.
Repairs and repainting are needed more often, but anodizing and powder coating processes are more cost–effective. If your project needs UV rays and element protection, consider other processes over painting.
| Metal Finishing Process | Advantages | Disadvantages | Thickness | Application Materials |
| Anodizing | Corrosion resistance, thicker coatings | Limited coloristic benefits | Varies, can achieve thicker | Aluminum, titanium, magnesium |
| Electroplating | Wide range of materials, cost-effective, aesthetic purposes | Time-consuming, thin coatings, affected by environmental factors | 0.1mm (0.004in.) | Copper, zinc, various metals |
| Powder Coating | Robust durability, cost-effective, decorative enhancement | Difficulty in color duplication, color consistency issues | N/A | Aluminum, steel |
| Painting | Accessible, versatile, long-term protection | Low durability, UV light sensitivity, corrosion resistance | N/A | Various materials |
Comparison of Metal Finishing Processes
Conclusion
You now know “what is anodizing”. You understand how it works and the types available. Complex components like aircraft parts need special consideration. Different materials require varying security levels, and balancing costs and results is crucial in order to ensure maximum safety.
You need all this information to decide and must compare anodizing, electroplating, and powder coating. Determine the best option for aesthetic appeal and durability.
KDMFab offers custom anodizing services catering to both professionals and home users, for more information on this service please visit the website.