
This article provides a discovery about what’s the softest metal on earth. In general, cesium has unique properties and stands out among the softest metals.
The world’s softest metal position on the periodic table is a scientific fact and a gateway to understanding its versatility. In any case, it is typical in healthcare & advanced tech. This article uncovers what are the softest metals and their use in diverse industries.
What’s the Softest Metal on Earth?
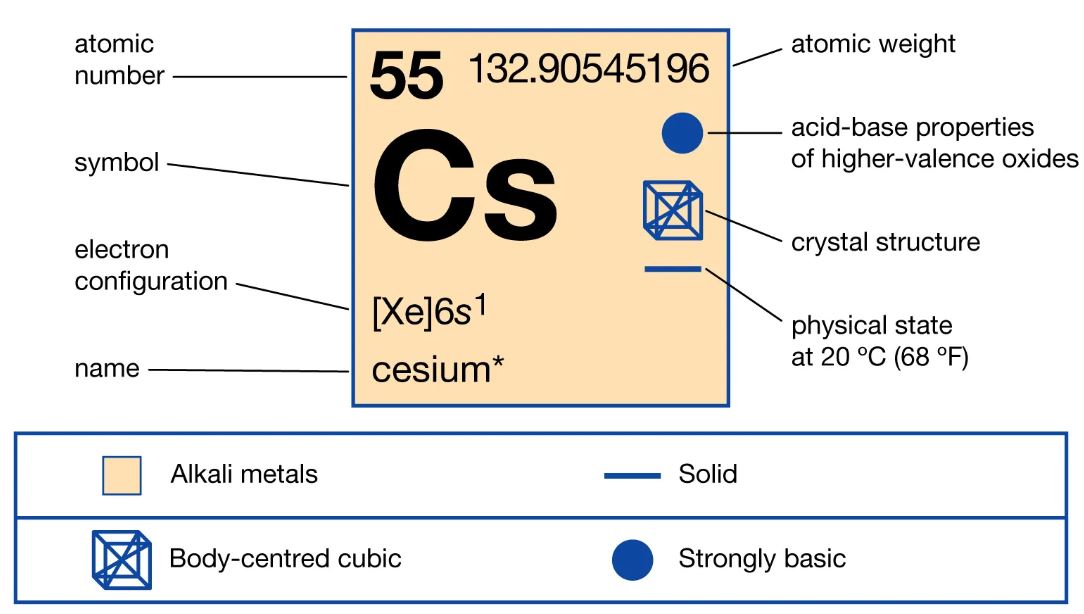
What is the softest metal on earth in periodic table, The Periodic Table lists cesium as the softest metal on earth. Overall, its symbol is Cs, and its atomic number is 55. It has a remarkable softness level and is easy to cut with a knife. In any case, the softest metals have a low melting point.
Meanwhile, cesium transitions into the liquid state slightly above room temperature. The metal stands out as the softest metal in alkalis.
In the meantime, other soft alkali metals include francium, potassium, and sodium. These alkali softest metals exhibit varying softness, suitable for scientific & tech settings.
Cesium: The Softest Metal on Earth
Robert Bunsen & Gustav Kirchhoff found what is the softest metal in 1860. In general, they discovered cesium through innovative spectral analysis. In any case, the discovery of what is the softest metal marked a significant leap in chemistry.
Its name reflects its striking blue lines within the spectrum. Cesium history is interlinked with the advent of spectroscopy. In general, the spectroscopy technique was vital in identifying cesium’s unique characteristics.
Overall, cesium is a scientific mystery among the alkali metals. The mineral is the answer to what is the softest metal and has deposits in Manitoba & Canada.
You will find that its softness is what makes it so remarkable. Its unique properties make it an element of interest across numerous fields.
Physical Properties of the Softest Metal on Earth
This section uncovers why cesium’s melting point is low and is a liquid at room temperature. In general, its features are fascinating as they find uses across different settings. Above all, cesium suits science & tech research on what are the softest metals.
Cesium Melting Point
Cesium turns into a liquid at 28.5 degrees Celsius or 83.3 degrees Fahrenheit. Overall, it has a low melting point due to its large atomic structure and weaker atomic bonds.
Meanwhile, this feature distinguishes cesium from the softest metals list and shapes its use in diverse settings. In simple words, cesium is a unique metal that challenges other metals in researching what are the softest metals.
Cesium Softness
Imagine a metal so soft that slicing it feels like cutting through butter. In any case, cesium’s unique softness is due to its large atomic size. In simple terms, it has weaker metallic bonds that allow the atoms to glide past each other with little resistance.
This feature distinguishes cesium from the softest metals list and influences its applications in various industries. Above all, this softness is what makes cesium an element of interest and value.
Cesium Density
Cesium has a low density of 1.93 grams per cubic centimeter. In other words, it is lighter than many other metals. The hardest and softest metal features an atomic size that can spread out atom arrangement to contribute to its density. In any case, density influences the hardest and softest metal in various applications.

Cesium Color & Appearance
Cesium has a unique silvery-gold color with a subtle blue tint. This appearance sets it apart from the list of metals hardest to softest. However, its interaction with air can make it dull. Its distinct look offers visual appeal and reflects its chemical properties. In any case, cesium’s color and appearance results from its reactivity.
Cesium State at Room Temperature
Cesium can turn into a liquid at room temperature. Overall, this feature results from its low melting point. It has unique characteristics that are rare among the list of metals hardest to softest due to its liquid state at room temperature.
In the meantime, it is critical to store cesium properly. This softest metal in the periodic table has distinctive features in the metal industry.
Cesium Thermal Expansion
Cesium is the softest metal in the periodic table that expands when you heat it due to its atomic makeup. It has a high thermal expansion to suit uses in tech & science. In other words, the softest metal in the world can shape shifts in fluctuating temperatures.
This aspect is a critical feature and is essential for diverse uses. Understanding how cesium reacts to heat helps design & leverage its features in various industrial settings.
Cesium Ductility
The softest metal in the world is ductile since you can stretch it into wires. In general, this feature is helpful in numerous settings. Besides its extreme softness, the softest metal on earth has high ductility and is suitable for tech & science. Overall, it blends softness & ductility for an exciting and practical element on the periodic table.
Cesium Electrical Conductivity
Cesium is also an excellent conductor of electricity. In the meantime, it stands out for its softness and conductive properties. In simple words, its electrons move freely to allow electrical flow. The softest metal on earth has excellent conductivity that adds to its value as a critical element in technology & science.
Cesium Photoelectric Effect
The world’s softest metal excels in exhibiting the photoelectric effect. In other words, it can release electrons when light hits its surface. In any case, it suits photoelectric settings like converting light into electrical signals since it is susceptible to light. The world’s softest metal property suits photoelectric cells and sensors.
Chemical Properties of the Softest Metal on Earth
Here, you will uncover what’s the softest metal on earth and how high reactivity & strong electro-positivity influence its benefits. Its chemical traits are vital for grasping its uses. Overall, the softest metal on earth finds applications in medicine & science.
Cesium Compounds
The softest metal on earth forms numerous compounds, including cesium chloride in medicine & research and cesium hydroxide. For instance, cesium carbonate is typical in organic chemistry labs, while cesium nitrate is common in pyrotechnics.
Cesium’s compound diversity demonstrates its versatility and reliability across different fields. Meanwhile, each compound serves specific purposes and showcases the adaptability of the softest alkali metal in science and technology.
Cesium Complexes
The softest alkali metal forms complexes from cesium ions encased with various molecules. A good example can be the cesium crown ether complexes. In any case, these complexes are essential in research for their unique ion encapsulation capabilities.
The softest alkali metal feature of forming such complexes extends its utility in chemical analysis and separation methods.
Cesium Halides
Metals from softest to hardest form various halides with halogens such as cesium fluoride, chloride, bromide, and iodide. In general, each halide has unique properties and uses. For instance, cesium fluoride provides fluoride ions in organic chemistry. On the other hand, cesium chloride suits medical and centrifugation settings.
Cesium Oxides
Metals from softest to hardest create various oxides with distinct properties and uses. Typical cesium oxides include cesium monoxide, peroxide, and superoxide. Cesium monoxide is vital in electronics for its conductivity, while cesium peroxide is critical in oxygen production. Meanwhile, cesium superoxide suits air purification.
Cesium Isotopes
The softest metal on Earth has various isotopes with diverse characteristics. In general, cesium-133 is the most stable cesium isotope and is typical in atomic clock precision.

On the other hand, cesium-137 stands out among its radioactive isotopes for its applications in medical treatments and industrial radiography. Overall, stable and unstable isotopes find applications in different settings. The isotope diversity proves the softest metal on earth influence in various science & tech settings.
Cesium Electro Positivity
Cesium exhibits remarkable electro-positivity since it is highly reactive. In general, this property influences its ability to form positively charged ions.
Its features are crucial in chemistry and medical treatments among the softest metals list. Overall, electro-positivity shapes cesium’s chemical features & applications.
Cesium Alloys
Cesium has a low melting point and exceptional thermal conductivity among the softest metals list. Thus, combining it with other metals creates unique alloys.
Meanwhile, ongoing research explores its potential in various industrial alloys as it allows adaptability in diverse fields.
Cesium Radioactive Decay
Cesium-137 isotope radioactive decay plays a significant role in various applications. Overall, the softest metal on earth can undergo beta decay and transform into barium-137. Hence, it can emit beta particles and gamma rays.
This unique property is critical in nuclear power plants for energy production and medicine in treatments & diagnostic procedures.
Extraction and Production of Cesium
You can extract the softest metal on earth as a byproduct of lithium production from pegmatite ores or pollucite ore.
In general, the process involves mining, mineral processing, and chemical separation. This subheading explores how this fascinating metal works.
Location of Cesium Deposits
Cesium deposits exist near lithium deposits in different parts worldwide. Popular regions include the Bikita pegmatite field in Zimbabwe, the Greenbushes Lithium mine in Australia, and the Tanco Mine in Canada.
In any case, the softest metal in periodic table deposits contains pollucite minerals. You might also encounter cesium in natural brine deposits, such as Clayton Valley, Nevada.
Cesium Mining Process
The mining process for cesium involves extracting it from mineral pollucite deposits. First, miners identify suitable cesium-rich residues in different geological settings. Hence, they can extract the ore through drilling and blasting.
The ore undergoes crushing and processing to remove impurities and isolate cesium-rich minerals. Meanwhile, the next step would be to refine these minerals to obtain cesium compounds like chloride or hydroxide. Further purification processes allow you to attain high purity in the softest metal in periodic table.
Cesium Initial Processing
The initial processing of cesium-rich ore involves several essential steps to extract and concentrate valuable cesium compounds. Simply put, you must crush & grind it into smaller particles after obtaining its ore from the mining site.
Hence, various chemical processes can help separate the softest metal in the world from other minerals and impurities. In any case, typical methods for removing impurities include flotation, leaching, and precipitation.
Moreover, further refining and purification processes ensure high-quality cesium products for various industrial applications like drilling fluids and atomic clocks.
Cesium Chemical Separation
This stage uses two standard methods, including solvent extraction and ion exchange. It employs an organic solvent to extract the softest metal in the world in the aqueous step.
On the other hand, ion exchange involves using ion-exchange resins to swap cesium ions for other ions present in the solution. These techniques help to isolate pure cesium compounds for quality cesium-based products.
Cesium Compounds
The softest metal in the world forms compounds that encompass diverse chemical substances containing cesium as a primary constituent.
Overall, these compounds exhibit unique properties due to their distinctive characteristics. One typical example is cesium chloride. In general, Cesium chloride forms a cubic crystal lattice structure and finds application in ultracentrifugation.
The softest alkali metal compounds suit chemistry research, electronics, and medicine. On the other hand, cesium iodide suits scintillation detectors for its excellent radiation detection. Meanwhile, cesium carbonate (Cs2CO3) is a base & organic synthesis catalyst.
Purification
Several meticulous steps occur in cesium purification to obtain a pure metal. The process involves crushing and milling the pollucite to reduce the particle size. Next, it undergoes acid leaching to dissolve cesium compounds.

Meanwhile, the resulting solution goes through various chemical processes, including precipitation and ion exchange chromatography.
One crucial aspect is eliminating impurities like sodium and potassium through multiple recrystallization steps. In addition, the softest metal in the world undergoes high-temperature reduction using calcium or lithium.
Elemental Cesium
Elemental cesium has a low melting point. Therefore, it is one of the few metals retaining the liquid state at or above room temperature. You can store the softest metal in the world in mineral oil to prevent its reactivity with air and moisture. Its remarkable properties make it invaluable in numerous scientific and tech endeavors.
Recycling & Recovery
Recycling & recovering the world’s softest metal is vital for sustainable use and conservation. The process involves extracting cesium from various sources, including spent nuclear fuel and industrial waste streams.
In general, it reduces waste and minimizes the environmental impact of cesium production. This sustainable approach ensures that cesium benefits various industries while preserving valuable resources.
Applications of the Softest Metal on Earth
The softest metal in the world has diverse applications due to its unique properties. Overall, you can find it in atomic clocks, which use vibrations to regulate time. Meanwhile, it is typical in drilling fluids for oil extraction and scientific research for its sensitivity to electromagnetic fields. Here are the uses of the softest metal in the world.
Nuclear & Isotope Applications
Cesium’s nuclear & isotope applications are a critical radiation source for cancer treatment. In general, cesium suits nuclear reactors for controlling the fission process.
The world’s softest metal has isotopes that help date archaeological artifacts and study environmental processes. In the meantime, its unique properties make it critical in various scientific and industrial applications for advancements in tech & research.
Chemical & Medical Use
Its chemical & medical uses are diverse & impactful. For instance, it is a critical catalyst in organic synthesis and aids in creating various compounds in the chemical industry.
In any case, cesium compounds are helpful for cancer therapy to target and destroy malignant cells. Its unique properties suit medical applications since it contributes to advancements in chemistry and healthcare.
Centrifugation Fluids
The world’s softest metal forms centrifugation fluids that suit particle separation in various settings. In any case, typical centrifugation fluids include saline solutions. Its density gradient may vary based on your specific application.
Selecting the proper fluid ensures accurate results in biology and clinical diagnostics. In the meantime, cesium centrifugation fluids enhance the effectiveness of the processes for successful research & experiments.
Electric Power
Electric power fuels homes, businesses, and technological innovations. In general, you can generate this power through various methods using cesium metal.
Typical methods include clean nuclear and renewable energy sources such as wind & solar. Hence, you can transmit the electricity from the softest metal in periodic table through extensive networks, power lines, and outlets.
Atomic Clocks
Atomic clocks are essential in timekeeping since they use atom vibrations to showcase time. In general, cesium & rubidium atoms use vibrations to define seconds. These clocks are vital in GPS, telecommunications, and space exploration.
In simple terms, the softest alkali metal ensures precise timing to billionths of a second. Thus, they have revolutionized global synchronization, impacted finance & science, and boosted innovation.
Petroleum Exploration
Petroleum exploration involves a complex process of searching underground oil reserves. Overall, geologists & geophysicists use advanced cesium techs to locate potential oil fields beneath the Earth’s surface.
This exploration is crucial for meeting global energy demands. In any case, the world’s softest metal uses cutting-edge tech and expertise to identify suitable drilling sites and extract valuable petroleum resources.
Space Applications
The softest metal on earth finds applications in various technologies and activities, including satellite communication, navigation, weather forecasting, Earth observation, and scientific research. In general, these activities are critical for global communication, climate change monitoring, and accurate GPS navigation.

Scientific Research
Scientific research using the softest alkali metal uncovers a fascinating niche. Overall, it involves forming hypotheses, conducting experiments, and analyzing earth’s data.
The world’s softest metal scientific research spans various fields, like biology, physics, chemistry, and astronomy. Overall, research drives innovation for tech advancements.
Optoelectronics
Optoelectronics is a fascinating field that merges optics and electronics. In any case, it focuses on devices that can control and convert light into electrical signals and vice versa. The softest metal in periodic table finds applications in optical fibers for telecommunications, LEDs in displays, and laser diodes in barcode scanners.
Cesium in Science & Technology
Cesium finds diverse applications in science and technology for its unique properties suitable for various fields. It holds a prominent place in atomic physics.
In general, the world’s softest metal atomic clocks offer exceptional accuracy using the precise resonance frequency of cesium atoms. These atoms are the standard for defining the SI second in the watches. Meanwhile, it provides accuracy for GPS systems, telecommunications, and scientific experiments.
Overall, the softest metal on earth is vital in ion propulsion systems as a propellant for spacecraft. It ionizes & expels the propellant at high velocities to generate thrust. Thus, this tech enables deep-space missions like those exploring outer planets and asteroids.
Meanwhile, photoelectric cells harness their photoemission properties in devices like digital cameras and solar panels. In general, the world’s softest metal is a reference for precise wavelength calibration in spectroscopy.
Its spectral lines are a critical benchmark for analyzing various elements in distant stars and galaxies. Above all, cesium is indispensable in advancing science and technology with multiple applications.
Factors that Determine Metal Softness
Understanding what influences the softness of metals is crucial. Overall, several factors affect a metal’s malleability and ductility. They include crystal structure and temperature since these factors shape the metal’s mechanical properties.
Type of Metal
The type of metal is a fundamental factor that determines its softness. Different metals have varying levels of malleability and ductility. For example, metals like cesium, gold, and silver are exceptionally soft and easy to shape.
In contrast, metals like steel and titanium are more complex and less malleable. The softest metal on earth has an atomic structure and bonding that contribute to its unique mechanical properties.
Interstitial Atoms
Interstitial atoms in the softest metal in periodic table occupy the spaces or interstices between the particles of a crystal lattice. Overall, these interstitial sites are smaller than the atoms with unique material properties. Interstitial atoms can influence the metal’s mechanical, electrical, and thermal properties.
For example, adding hydrogen atoms to metals can enhance their ability to absorb hydrogen gas. The presence and arrangement of interstitial atoms are vital in tailoring the softest metal in periodic table for specific applications.
Grain Size
Grain size is the size of individual crystalline grains in a polycrystalline material like metals or ceramics. In any case, it determines the mechanical properties of the softest metal in periodic table. Smaller grains lead to increased strength and hardness, while larger grains can enhance flexibility.
In general, engineers and metallurgists can control grain size through heat treatment processes like annealing or quenching. Hence, they can tailor the softest metal on earth to meet specific requirements for various applications.
Cold Working
Cold working involves shaping metals at room temperature. In general, it subjects a metal to mechanical stress, such as rolling, drawing, or extrusion. For that reason, it can change shape and improve its mechanical properties.
The softest metal in periodic table can undergo cold work to boost hardness, strength, and surface finish. It is a typical solution in industries like automotive and aerospace to produce precision components like fasteners, shafts, and tubing.
Thermal History
Thermal history is the temperature-related changes a material undergoes throughout its processing and use. In any case, it is a critical factor in determining a material’s properties and performance. Understanding the thermal history of the softest metal on earth helps engineers and scientists predict its behavior in various conditions.
For example, metals heat-treated to specific temperatures may exhibit improved strength or hardness. In contrast, exposure to high temperatures can lead to thermal stress and degradation in the softest metal on earth.
Impurities & Alloying
Impurities impact the properties of materials and can alter the mechanical, electrical, or chemical properties of the softest metal on earth. For example, adding small amounts of carbon to iron produces steel.
In the meantime, controlled doping introduces impurities into semiconductors to modify their conductivity. Thus, understanding how impurities affect cesium is essential for tailoring its characteristics to specific applications.
Crystal Structure
The crystal structure of a material defines its atomic arrangement and influences its properties. In general, crystals can have various forms, such as cubic, hexagonal, or tetragonal. This arrangement determines the mechanical, thermal, and electrical features of the softest metal on earth.
For example, diamonds’ crystal structure makes them one of the most complex materials, while graphite’s layered structure imparts its lubricating properties.
Electron Configuration
Electron configuration is the electron distribution in an atom’s energy levels and orbitals. Overall, it is a crucial concept in chemistry as it determines an element’s chemical properties and reactivity.
The electron configuration follows specific rules to dictate the filling order of orbitals. Understanding electron configurations in the softest metal on earth allows chemists to predict how atoms bond and form molecules. Above all, it provides valuable insights into the behavior of the softest metal in the world in chemical reactions.
Metallic Bonding Strength
Metallic bonding strength is a fundamental property of metals that defines their ability to hold atoms together in a solid structure. In general, it arises from sharing electrons among a metal’s positively charged atomic nuclei across the softest metals list.
The strength of metallic bonds varies among different metals and depends on factors like the number of valence electrons and nuclear size. In any case, metals with more valence electrons and smaller atomic sizes have stronger metallic bonds. This bonding strength contributes to the melting and boiling points of the softest metal in the world.
Atomic Size
Atomic size is crucial in understanding the softest metal in the periodic table. It refers to the atom size, indicating the distance from the electron shell to the nucleus. Atomic size increases as you move down a group in the periodic table.
In addition, the atomic size decreases across a period from left to right. This radius influences various properties, including reactivity, chemical bonding, and physical characteristics across the softest metals list.
Cesium Comparison with Other Metals
Cesium differs from other metals in its remarkable softness. In addition, it has a low melting point and high reactivity to suit various applications. Cesium requires careful handling since it has extreme sensitivity to moisture.
Cesium vs. Gold
Is gold the softest metal on the softest metals list? Overall, cesium features extreme softness and a low melting point. In contrast, gold is a dense and malleable metal with a striking yellow color. Cesium is highly reactive and requires careful handling.
Meanwhile, gold is unreactive and corrosion-resistant. These differences in physical and chemical properties contribute to their unique uses in various industrial and scientific applications. Thus, the response to gold the softest metal, is no.
Cesium vs. Lead
Cesium has extreme softness and reactivity and is typical in propulsion systems, photoelectric cells, and atomic clocks. On the other hand, lead is a heavy and dense metal that is typical in construction and batteries.
The softest alkali metal offers unique properties in scientific & tech fields, while lead has durability and corrosion resistance. However, lead is toxic and needs careful handling. The softest metal in the world requires careful storage and handling due to its reactivity.
Cesium vs. Mercury
Cesium and mercury are typical in atomic clocks, photoelectric cells, and ion propulsion systems due to their exceptional reactivity. On the other hand, mercury is an ideal choice for its use in thermometers and barometers.
However, it is crucial to note that mercury has significant health and environmental risks due to its toxicity. In contrast, the softest metal on earth has no toxicity concerns.
Cesium vs. Lithium
Cesium and lithium are two alkali metals with distinct characteristics and applications. Cesium is the softest metal on earth and has exceptional reactivity. In contrast, lithium is lightweight and is typical in rechargeable batteries for smartphones & EVs.
Cesium vs. Aluminum
Cesium and aluminum are different in terms of their properties and applications. Cesium is the softest metal on earth and is highly reactive. In general, it can be a suitable choice in scientific research and atomic clocks.

On the other hand, aluminum is a lightweight and durable metal that is typical in construction and aerospace. The softest alkali metal has unique strengths and weaknesses. Cesium’s extreme reactivity suits scientific contexts, while aluminum’s versatility & strength are ideal for modern engineering and manufacturing.
Health and Environmental Impact of Cesium
The softest metal on earth has several health & environmental impacts. Exposure to cesium can be harmful since ingesting or inhaling cesium can lead to various health issues. Typical health concerns include nausea, diarrhea, and heart & liver damage.
Meanwhile, cesium contamination can have serious environmental impacts. Accidental spills or improper disposal can lead to soil & water pollution. In general, the softest alkali metal can impact the environment longer, hampering the food chain.
Stringent safety measures are vital when working with cesium. In addition, proper disposal methods are crucial to prevent environmental contamination.
Meanwhile, ongoing research focuses on understanding the world’s softest metal and its potential long-term effects on human health. Thus, researchers recommend regulations & safety practices to minimize the health & environmental risks.
Challenges in Handling Soft Metals
Handling soft metals presents several challenges that require careful consideration. Soft metals have unique properties that make them prone to several issues. First, the softest alkali metal is highly reactive to moisture and air.
In other words, the softest metal on earth can quickly corrode or form oxide layers. This reaction compromises their purity and performance. Thus, special handling procedures and controlled environments are essential to prevent contamination.
Second, soft metals are malleable and ductile. Their low hardness can lead to deformation or damage during processing and manufacturing.
Moreover, the softest metal in periodic table has a low melting point, necessitating precise temperature control during applications. Above all, soft metals applications require proper training and safety protocols to prevent accidents.
Conclusion
Cesium reigns as the softest metal on earth with its unique properties. They include extreme softness, low melting point, fascinating chemistry, and materials science elements. Most importantly, the softest metal in periodic table suits various industries, including electronics and atomic physics.
Visit KDM Fabrication to explore the diverse applications of the world’s softest metal. Discover how this remarkable element shapes the world and contributes to tech innovations. Unlock the potential of soft metals with KDM Fabrication today!
FAQ
- What makes cesium the softest metal on Earth?
Cesium’s unique status as the softest metal on earth results from its low melting point and the ease with which its atomic layers can slide past one another. In general, these characteristics result from its large atomic size and the weak forces holding its atoms. Therefore, cesium exhibits remarkable softness and stands out in the periodic table.
- Is cesium safe to handle every day?
Handling the softest alkali metal frequently is unsafe due to its extreme reactivity with air and water. In any case, it can catch fire quickly and explode in contact with water. Thus, ensure extreme caution and apply specialized equipment to prevent accidents.
- What are the environmental impacts of cesium?
Accidental releases of cesium isotopes from nuclear accidents can contaminate soil and water. Hence, the softest metal in the world may pose long-term health risks to ecosystems. Proper disposal measures are vital to mitigate these impacts.
More Resource:




